Celebrating the ICR’s achievements under Professor Paul Workman’s leadership

Professor Paul Workman steps down as Chief Executive of The Institute of Cancer Research, London, at the end of August after seven years in the role.
He has worked at the ICR since 1997, when he joined as Director of the Cancer Research UK Cancer Therapeutics Unit.
Here, we look back at a selection of the many ICR achievements during Professor Workman's hugely successful period at the helm of the organisation.
2013-14
2014-15
- We put in motion ambitious plans to transform our Sutton site into a world-leading life-science campus – The London Cancer Hub.
 The project, which is a partnership between the ICR and the London Borough of Sutton, is designed to create a vibrant community for cancer research, treatment, education and business enterprise.
The project, which is a partnership between the ICR and the London Borough of Sutton, is designed to create a vibrant community for cancer research, treatment, education and business enterprise.
- Our Centre for Cancer Imaging opened its doors to its first wave of researchers. This new leading-edge research facility was designed to promote multidisciplinary team working, drive the development of new imaging techniques, and accelerate progress in drug discovery.
- The ICR was ranked as the top higher education institution in the UK for the quality of its research, in the Times Higher Education league table compiled from the Research Excellence Framework. We were also ranked the leading higher education institution in the UK for the impact of our research on society.
2015-16
- Together with our partner hospital, The Royal Marsden NHS Foundation Trust,
 we unveiled a bold and ambitious new research strategy aimed at confronting the challenges of cancer evolution and drug resistance.
we unveiled a bold and ambitious new research strategy aimed at confronting the challenges of cancer evolution and drug resistance.
- The ICR’s efforts to promote the role of women in science were recognised with an organisation-wide Athena SWAN Silver Award. At the time we were one of only three research institutes to hold the award.
- In 2016 the ICR was among a select group of 12 institutions across the UK to be awarded a highly prestigious Regius Professorship, a rare award bestowed by Her Majesty the Queen to recognise high quality research at an institution. We were granted the first ever Regius Professorship of Cancer Research, in recognition of both the academic excellence and the real-world impact of our research – the ICR then named Professor Johann de Bono as the first Regius Professor of Cancer Research.
- NICE announced that abiraterone, the life-extending prostate cancer drug discovered at The Institute of Cancer Research, London, would be made available for men on the NHS earlier in the course of their treatment, without them needing to go through chemotherapy first. It was first approved for use on the NHS in 2012 as a standard treatment for advanced prostate cancer after chemotherapy.
2016-17
- The ICR and The Royal Marsden became the first institutions in
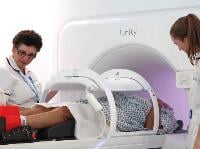 the UK to install a revolutionary MR Linac radiotherapy machine, opened by Sadiq Khan, the Mayor of London. A £10m grant from the Medical Research Council to the ICR funded the purchase of the MR Linac system – which combines an MRI scanner and a linear accelerator – and helped to finance its installation in a new facility at The Royal Marsden.
the UK to install a revolutionary MR Linac radiotherapy machine, opened by Sadiq Khan, the Mayor of London. A £10m grant from the Medical Research Council to the ICR funded the purchase of the MR Linac system – which combines an MRI scanner and a linear accelerator – and helped to finance its installation in a new facility at The Royal Marsden.
- The ICR and Imperial College London marked the start of a new formal partnership with the launch of the Cancer Research Centre of Excellence, harnessing the complementary expertise of both partners. Professor Paul Workman was announced as Director of the Centre.
- The ICR earned more invention income from its research than any other higher education institution in the UK. The ICR regularly ranks first among UK higher education institutions for income from intellectual property in figures adjusted for size – and in some years even came top in absolute income.
2017-18
- The ICR was awarded
-tmb-0874x492.tmb-0thumb200.jpg?sfvrsn=ed975069_2) a Queen’s Anniversary Prize for pioneering discoveries in precision medicine for cancer. The Queen’s Anniversary Prize acknowledges the ICR for its outstanding contribution to the discovery of new cancer drugs – including pioneering the transition from one-size-fits-all chemotherapy to targeted drug treatment. Since 2005 the ICR has discovered 20 new molecularly targeted cancer drugs and taken 11 into clinical trials.
a Queen’s Anniversary Prize for pioneering discoveries in precision medicine for cancer. The Queen’s Anniversary Prize acknowledges the ICR for its outstanding contribution to the discovery of new cancer drugs – including pioneering the transition from one-size-fits-all chemotherapy to targeted drug treatment. Since 2005 the ICR has discovered 20 new molecularly targeted cancer drugs and taken 11 into clinical trials.
- The ICR and Cancer Research UK established a major strategic collaboration with Merck, a leading science and technology company, to discover new cancer drugs. The new alliance, set up via the ICR’s Business and Innovation Office, takes a strategic joint approach to drug discovery in areas of shared scientific expertise. Three independent research projects were initially planned to discover new preclinical drug candidates.
- IMRT – a high-tech form of radiotherapy developed by researchers at the ICR and The Royal Marsden that shapes radiation beams to tumours – was shown in a trial led by the ICR and The Royal Marsden to dramatically improve outcomes for patients with prostate cancer. The long-term follow-up study showed that the treatment was safe and that 71% of patients with prostate cancer were alive and disease free five years after treatment. The results have changed clinical practice, with IMRT becoming the standard of care at major cancer centres in the UK and worldwide.
- The ICR and the University of Cambridge together received major funding for a new paediatric brain tumour centre as part of a £45 million new investment from Cancer Research UK and the Department of Health and Social Care. The Cancer Research UK Children’s Brain Tumour Centre of Excellence is co-led by Professor Paul Workman at the ICR and Professor Richard Gilbertson at the University of Cambridge.
- The ICR ranked first in the world for the percentage of publications that are cited in patents – a measure of how innovative our research is – in an independent 2018 evaluation funded by the European Commission, called U-Multirank. We ranked fourth in the world for the proportion of publications that are highly cited – with over 25 per cent of our publications reaching the top 10 per cent of frequently cited articles in their field. Overall, we ranked in the top 30 higher education institutions out of more than 1,200 worldwide. The ICR continued to rank highly in U-Multirank in following years.
- The PARP inhibitor olaparib was approved by the US Food and Drug Administration (FDA) for patients with BRCA-mutant advanced breast cancer – the first cancer drug in the world to target inherited genetic faults. Olaparib was developed as a genetically targeted cancer drug following landmark research led by scientists at the ICR, who in 2005 demonstrated that cancer cells with BRCA1 or BRCA2 mutations were very sensitive to PARP inhibitors. Those findings followed the isolation of the BRCA2 gene in 1995 by scientists at the ICR. Researchers at the ICR and The Royal Marsden played leading roles in the clinical evaluation of olaparib.
- As a leading example of our fundamental research, ICR scientists unveiled in the journal Nature a detailed cryo-EM structure showing how the DNA code is read by RNA polymerase III – a protein that is hijacked in cancer. This discovery also illustrates the importance of structural biology – determining the 3D shapes of the cell’s proteins – that the ICR enabled by its investment in cutting edge Cryo-EM equipment both in-house and at the Francis Crick Institute.
2018-19
 The ICR and Imperial College London established an ambitious new Convergence Science Centre with £13 million in funding over five years from Cancer Research UK, which awarded it Major Centre status. The vision is to combine complementary expertise in the engineering, physical, mathematical, data, life and medical sciences in the two organisations to tackle major challenges in understanding, detecting and treating cancer. Professor Paul Workman led the application and was appointed as the Founding Director of the Centre.
The ICR and Imperial College London established an ambitious new Convergence Science Centre with £13 million in funding over five years from Cancer Research UK, which awarded it Major Centre status. The vision is to combine complementary expertise in the engineering, physical, mathematical, data, life and medical sciences in the two organisations to tackle major challenges in understanding, detecting and treating cancer. Professor Paul Workman led the application and was appointed as the Founding Director of the Centre.- An ICR report on access to the latest cancer drugs sparked debate across the media, online and among patients and policy makers about how to accelerate entry of innovative new treatments into the NHS. The report was followed by a manifesto outlining possible solutions to overcome the challenges in cancer drug access, and later by a peer-reviewed publication. The ICR convened a round table of representatives from industry, academia, charities and the NHS, which led to the agreement of a series of consensus statements aimed at delivering for cancer patients.
- The ICR launched the institution’s Six Values. Developed together as an organisation, the ICR Values make clear how everyone at the ICR, across research and professional services, should work to meet our mission – to make the discoveries that defeat cancer. The commitment to ‘value all our people’ would then be central to the ICR’s future work on equality, diversity and inclusion – which includes our Beyond the Statements project aimed at tackling racial inequality at the ICR and beyond.
- The ICR was recognised for its many successes in drug discovery through the award of blue plaques in Sutton and Chelsea, under the Royal Society of Chemistry’s Chemical Landmark scheme. The plaques mark the role the ICR has played in cancer drug discovery from the 1950s until the present day – including the discovery of chemotherapy drug carboplatin, prostate cancer drug abiraterone and the genetic targeting of olaparib for ovarian and breast cancer.
2019-20
- Monte Rosa Therapeutics, a
 company formed as a spinout from Cancer Research UK-funded science at the ICR, was publicly launched. The company raised $32.5m in venture capital funding based on a library of innovative anti-cancer compounds designed at the ICR, which work like molecular glues that stick proteins to each other – leading to the elimination of cancer-causing targets. The company then went on to secure $96m of ‘series B’ financing from investors to support the further development of its drug pipeline and generated a further $222 million through an IPO which saw them listed on the Nasdaq stock exchange.
company formed as a spinout from Cancer Research UK-funded science at the ICR, was publicly launched. The company raised $32.5m in venture capital funding based on a library of innovative anti-cancer compounds designed at the ICR, which work like molecular glues that stick proteins to each other – leading to the elimination of cancer-causing targets. The company then went on to secure $96m of ‘series B’ financing from investors to support the further development of its drug pipeline and generated a further $222 million through an IPO which saw them listed on the Nasdaq stock exchange.
- The ICR recruited 24 new research Team Leaders, growing our capacity in key strategic areas. Among our new researchers was Professor Axel Behrens, who will provide world-class leadership for our ambitious Cancer Research UK Convergence Science Centre with Imperial College London.
- The ICR ranked top for collaboration in a 2020 report published by the UK patent office on higher education institution patent filings. The UK Intellectual Property Office data showed that the ICR filed some 92% of patent applications with an industry partner, compared with an average of about 60% across other UK higher education institutions.
- The London Borough of Sutton and the ICR announced plans for a new life-science incubator at The London Cancer Hub in Sutton, allowing companies the opportunity to work directly alongside the ICR’s world-class scientists. The new Innovation Gateway will provide a home for start-ups and spin-outs, as well as potentially satellite bases for larger biotech, med-tech, pharmaceutical, and digital or data companies. This development followed the London Borough of Sutton investing £28 million to purchase land on the site.
- The ICR was recognised in the 2020 Budget as a world-leading specialist institution that would receive a share of £80 million extra Government funding over the next five years.
- Approval by the US Food and Drug Administration (FDA) of the genetically targeted PARP inhibitor drug olaparib for men with advanced prostate cancer with BRCA mutations, following major trials led by ICR researchers.
- A trial led by the ICR showed that chemotherapy halves the risk of a rare form of kidney cancer recurring after surgery. In the largest ever trial conducted in the disease worldwide, upper tract urothelial cancer patients given chemotherapy after surgery saw the risk of their cancer returning or spreading reduced and were much more likely to live cancer free for three years or more. The new results are set to change clinical practice around the world.
- A major phase III trial of capivasertib, a cancer drug discovered following collaborative work between the ICR and partners Astex Therapeutics and AstraZeneca, began in breast cancer. A total of three Phase III trials are now underway with this AKT inhibitor.
- In research led by the ICR, a one-week course of radiotherapy in fewer but larger daily doses was found to be as safe and effective as standard three-week therapy for women following surgery for early stage breast cancer. The shorter treatment, which is likely to be practice-changing, was taken up quickly by many hospitals to help reduce demands on the NHS during the COVID-19 pandemic.
2020-21
- The ICR’s £75m
 Centre for Cancer Drug Discovery opens to researchers. Scientists at the new pioneering centre for 'Darwinian' cancer drug discovery hope that their approaches will lead to long-term control of cancer, as well as cures, by overcoming cancer evolution and drug resistance. Located on the Sutton site of the ICR, the state-of-the-art facility houses the ICR’s drug discovery scientists and the Centre for Evolution and Cancer – bringing together around 300 leading researchers in a single collaborative space.
Centre for Cancer Drug Discovery opens to researchers. Scientists at the new pioneering centre for 'Darwinian' cancer drug discovery hope that their approaches will lead to long-term control of cancer, as well as cures, by overcoming cancer evolution and drug resistance. Located on the Sutton site of the ICR, the state-of-the-art facility houses the ICR’s drug discovery scientists and the Centre for Evolution and Cancer – bringing together around 300 leading researchers in a single collaborative space.
- A new Unit focusing on cancer prevention research and strategies was launched at the ICR and Imperial College London. The Cancer Epidemiology and Prevention Unit (CEPRU) is a joint initiative between the two leading institutions. Its vision is to become a global leader in advancing research on the causes of cancer, and implementing effective prevention strategies through collaborations.
- The ICR announced plans to establish an Integrated Pathology Unit (IPU) in partnership with The Royal Marsden. Focusing on digital pathology, the IPU will bring together molecular pathology research across the joint institution. Linked to artificial intelligence technology, digital pathology is set to transform the way we understand, diagnose and characterise cancer, and is helping to shape how cancer is treated. The IPU will ensure all pathology images across different cancer pathways are captured, stored and shared as digital images, enhancing both research and patient care.
- A practice-changing clinical trial led by Professor Andrew Tutt shows benefits of the PARP inhibitor olaparib against early-stage breast cancer with inherited BRCA mutations. Adding olaparib for one year following standard treatment greatly reduced the rate of recurrence.
- After a hugely challenging period, the ICR began to emerge strongly from the Covid-19 pandemic. The hard work and dedication of the ICR’s professional services, research staff and students ensured that laboratories were able to reopen with Covid-safe measures in place, and office-based colleagues have begun to return. Researchers contributed to the national scientific effort against Covid-19 throughout the period. The ICR’s Development Office launched a kick-start appeal, which helped to raise crucial funds to contribute to getting our research fully up and running again, so that we can make up for time lost due to the pandemic.
- The ICR launched a Centre for Translational Immunotherapy (CTI). The Centre brings together staff and students from the ICR and The Royal Marsden aiming to develop a greater understanding of how immunotherapy works and develop biomarkers to aid clinical trials and treatment. The CTI is one of several collaborative centres launched at the ICR over the past two years that bring together expertise from across the ICR and The Royal Marsden. Others include the Joint Sarcoma Research Centre, the Centre for Global Oncology, and the Centre for Paediatric Oncology Experimental Medicine.
- Fadraciclib, a CDK9/2 inhibitor discovered at the ICR in collaboration with the biotechnology company Cyclacel, entered a first-in-child study for paediatric patients with aggressive neuroblastoma. The drug reduces production of the NMYC protein that drives the disease and shows impressive activity in preclinical models on neuroblastoma. This achieves an ambition of Professor Paul Workman to discover a drug at the ICR that would be progressed into clinical trials for children with cancer.
- The Genome Damage Centre launched at the ICR. It is co-led by Professor Jon Pines, who was recruited as Head of the Division of Cancer Biology in 2014-15 and has since led a major refocusing of the Division, which aims to understand fundamental cell biological mechanisms involved in cancer – including DNA replication and repair, cell division, signalling, metabolism, and migration – and identify new targets for treatment.
- canSAR celebrated its 10th anniversary. Since its launch in 2011, canSAR has become the largest, public, cancer drug discovery resource in the world. This AI-enabled integrative knowledgebase was created by Professor Bissan Al-Lazikani and developed with Professor Paul Workman as co-investigator. It feeds into another ICR-led resource, Probe Miner, the large-scale, objective nature of which is very complementary to a further resource – namely the Chemical Probes Portal overseen by Professor Workman, which is based on expert opinion. Chemical probes are vital reagents to determine the effects of modifying the function of proteins in biomedical research and ICR researchers discover chemical probes and help develop best practice for their use.
- An innovative first-in-class drug discovered at the ICR, NXP800, is licensed to the newly formed biotechnology company Nuvectis which plans to initiate the Phase I clinical trial in the Drug Development Unit at the ICR and The Royal Marsden by the end of the year. The drug, which inhibits the Heat Shock Factor-1 (HSF1) pathway, was discovered following 14 years of research originated and led by Professor Paul Workman. It shows promise in preclinical models of ovarian clear cell cancer.
- A drug discovered at the ICR that could counteract drug resistance in patients with cancers including acute myeloid leukaemia (AML) entered a phase I clinical trial at The Royal Marsden. Licensed to the UK biotech company Ellipses Pharma, EP0042 is a dual inhibitor of cancer-driving proteins from the FLT3 and Aurora families – meaning that it blocks the activity of both targets at once. EP0042 shows promise in models of resistance to FLT3-selective inhibitor drugs. In addition to drug resistant AML, it also has potential as a future treatment for other cancers including the childhood cancer neuroblastoma. More broadly, the ICR has made considerable progress in our overall research strategy goal of understanding and overcoming cancer evolution and drug resistance, including use of liquid biopsies to detect early changes and use of innovative drug combinations.
Colleagues from the ICR and beyond share their reflections on working with Professor Paul Workman - as both a scientist and a CEO.
Read the tributes