Image: ICR researchers Professor Alan Melcher, Professor Clare Isacke, Dr Pablo Nenclares and Dr Ben O’Leary
In 2008, only around one in 20 patients with advanced melanoma survived for five years beyond diagnosis. Today, around half of patients with this kind of cancer reach that milestone, largely due to immunotherapy.
“Survival was dismal,” recalls Professor Alan Melcher, consultant oncologist and Professor of Translational Immunotherapy at The Institute of Cancer Research, London, “but with immunotherapy things changed dramatically. Immunotherapy has been the biggest breakthrough in cancer treatment in the last two decades, but it’s still early days.”
So why has immunotherapy been so revolutionary, and how can we be confident that it will save even more lives from cancer in the future?
A new approach in an age-old battle
It’s long been known that people whose immune systems are compromised – such as transplant recipients – have a greater risk of some cancers.
This suggests that people with a healthy immune system have some level of in-built protection against cancer, that perhaps sometimes the immune system can search and destroy cancer cells before we even know they’re there. However, the fact that cancer is so common shows that this protective power is frequently outmatched by the disease.
The aim of immunotherapies is to empower the immune system in this battle of biology. Unlike traditional treatments that target cancer cells directly, immunotherapies tend to target immune cells, arming them to fight cancer for themselves. It’s a revolutionary approach that began with a brilliant discovery three decades ago.
A eureka moment across the pond
In the early 1990s, James P Allison, head of the Department of Immunology at University of Berkeley, California, was on a quest to understand the detailed workings of T cells. These cells are on the frontline of our immune response, patrolling the body for invaders, and eradicating them.
Professor Allison was studying a protein on the surface of T cells called CTLA-4. His painstaking work revealed that it acted as a brake on T cell activity – an in-built system checkpoint. Excited by this finding, Allison immediately questioned whether ‘releasing the brake’ might liberate the immune system to kill cancer cells, and he designed some experiments to test his theory.
Much to his astonishment, that’s exactly what happened. Professor Allison witnessed tumours in mice shrink and disappear in a matter of weeks when they were given an antibody to block (or ‘inhibit’) CTLA-4.
This discovery – for which Allison received a Nobel Prize – would bring cancer treatment into a new era. In 2010, a clinical trial of ipilimumab (a CTLA-4 inhibitor) in patients with metastatic melanoma was the first to report positive results for a cancer immunotherapy.
Immunotherapy has transformed the lives of many people with cancer over the last decade – including Graham, who was diagnosed with kidney cancer, but following successful immunotherapy was told he no evidence of the disease.
Image: Human melanoma cells growing in culture, showing DNA, mitochondria and endoplasmic reticulum (ER). Credit: Paul J.Smith & Rachel Errington. CC BY 4.0.
Transformational results
The success of CTLA-4 inhibition in melanoma was revolutionary. Since then, a host of immune checkpoint-inhibitor medicines have become available and part of the standard treatment strategy for an increasing number of cancers, including other difficult-to-treat types such as lung and oesophageal cancer. They have given thousands of cancer patients in the UK alone more precious years with their loved ones. Many can live a life with no evidence of cancer.
At the ICR, our researchers have played a vital role in generating the evidence needed to bring these drugs to patients. For instance, Professor Kevin Harrington led the UK arm of a pivotal trial of a checkpoint inhibitor called nivolumab. It involved 361 patients with head and neck cancer whose disease had worsened within six months of having chemotherapy. Patients in this situation had an average life expectancy of six months, which no treatments had been able to improve. Nivolumab doubled survival to one year, compared to further chemotherapy, for more than a third (35%) of patients and came with fewer side effects.
But success with any immunotherapy is not guaranteed. Some cancers are stubbornly resistant to the current drugs. Even in cancer types that can respond well, many patients don’t see a benefit.
So how can we deliver the promise of immunotherapies to more patients and more kinds of cancer? The answer undoubtedly lies in research.
Research will unlock the potential of immunotherapy
While immunotherapy is now a common cancer treatment, we know remarkably little detail about how it works, and even less about why it works in some people but not in others. The science is still relatively young, which means discoveries that bring the next generation of immunotherapies to more patients could be just around the corner.
It’s not going to be easy. Professor Melcher describes studying the immune system as like “shooting at a moving target” – it’s in constant flux. And it’s clear that the way cancers outwit the immune response is not the same in everyone or in every cancer type. We know some cancers use ‘cloaking devices’ to hide from the immune system, build physical defences around a tumour, or evolve to try and stay one step ahead of immune attack strategies.
That’s why, at the ICR, we’re working on multiple fronts too. Success will come from bringing discovery scientists from across disciplines together with clinical researchers and patients at our hospital partner The Royal Marsden. This is what our Centre for Translational Immunotherapy – led by Professor Melcher – is all about; to bring together everyone working on any aspect of these problems to share ideas and insights and accelerate the pace of discovery.
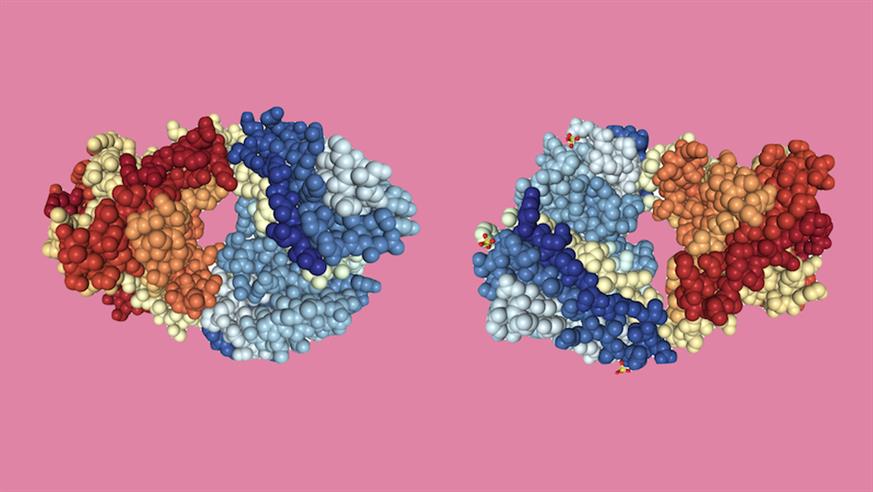
Image: Crystal structures of the Fab fragments of immunotherapy drugs nivolumab (left) and ipilimumab (right). Based on PDB structures 5GGQ and 6JC2. Structural images created using NGL viewer and RCSB PDB.
Wide-ranging research
At the ICR, our research has the potential to create tests that predict who will respond to immunotherapy, to unmask more cancers so that they become vulnerable to immune attack, to generate personalised vaccines tailored to an individual’s disease, and to optimise the timing and delivery of immunotherapy to make it even more effective.
For instance, Professor Clare Isacke’s team is investigating the way that breast tumours shield themselves from immune attack with a blockade of cells called fibroblasts. They are currently developing antibody-based therapies to try to destroy fibroblasts in the tumour environment, therefore exposing breast cancer to immune attack.
Dr Pablo Nenclares, a clinical oncologist undertaking a PhD at the ICR, is investigating the role that the immune system plays in the success of traditional treatments. He has looked back at blood samples from people who’ve received chemotherapy and radiotherapy for cancers caused by human papillomavirus (HPV) infection. He’s found that in patients whose cancers were successfully treated, there was clear evidence that the immune system had previously armed itself against HPV-infected cells. In contrast, in patients whose cancers did not respond to treatment, the immune system was unarmed against HPV.
More work is needed, but the results suggest that preparing and supporting the immune system before or during traditional treatments, could make them more effective. Monitoring immune changes during these treatments may also be a way to track how the patient’s cancer is responding.
Dr Ben O’Leary, a clinical scientist in the ICR’s Molecular Oncology group is leading the DART study, involving patients receiving immunotherapy for head and neck squamous cell carcinoma. By collecting blood and saliva at each treatment cycle, and tracking treatment progress, Dr O’Leary hopes to identify molecular markers in the patients’ samples that could in future help doctors create personalised treatment strategies for the best chance of success.
The world-class immunotherapy research going on across the ICR – linked by the Centre for Translational Immunotherapy – is driving progress towards the next generation of immunotherapies and bringing the hope of a cure to more people with cancer.
Immunotherapy is already transforming lives, but we need to untap its full potential. Our research plans are ambitious and need ongoing support. Please make a monthly donation to help patients live longer and to save many more lives.