Landmark Nature papers unveil ‘dark matter’ shaping cancer behaviour
October 2022
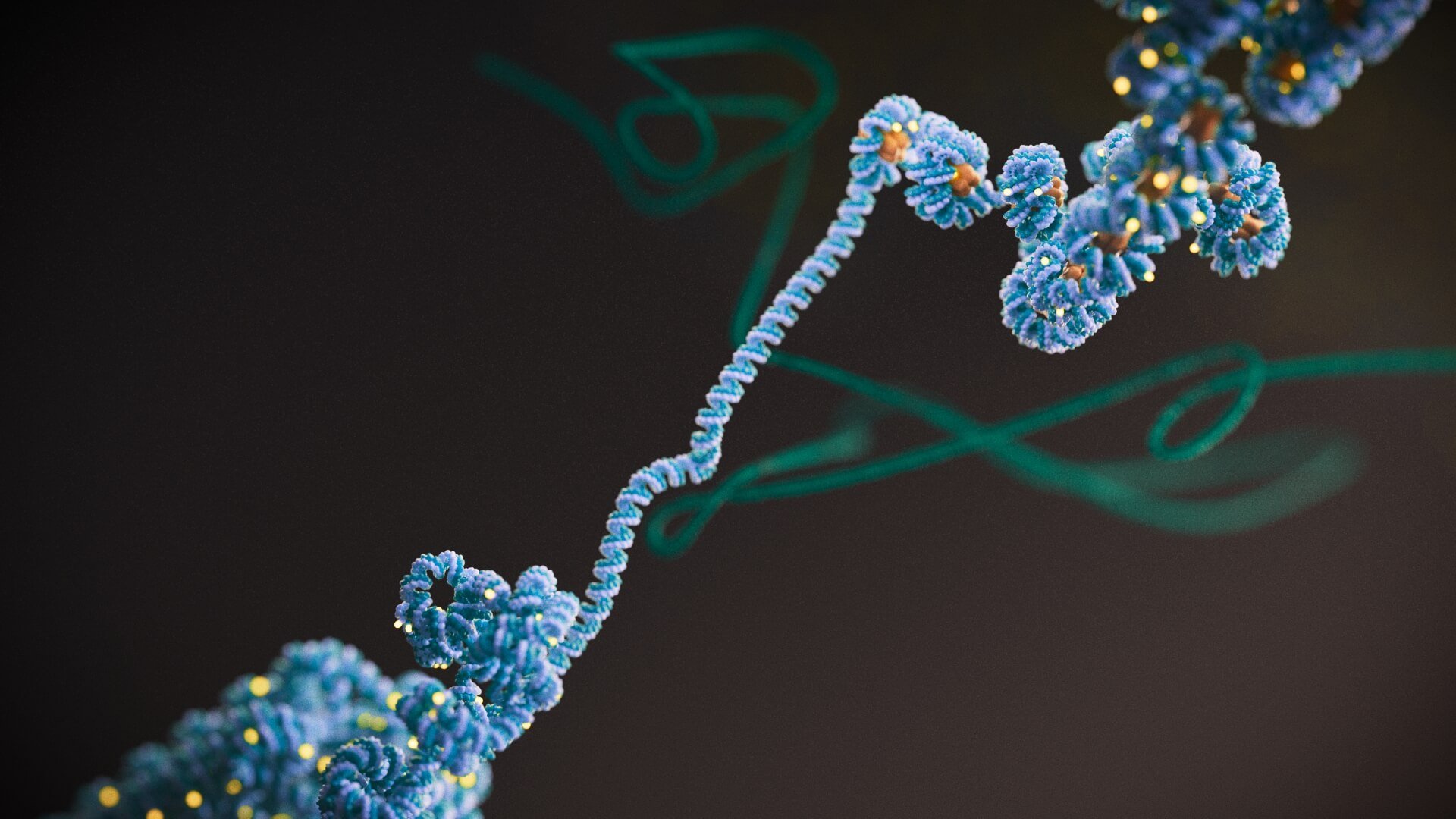
Image: DNA unwinding from histones. The 3D structure of DNA can influence which genes can be turned on and off. Credit: Phospho Biomedical Animation
Scientists at the ICR were responsible for two landmark studies that could ultimately change how we think about cancer and its treatment. They confirmed that an extra level of control over gene activity, known as epigenetics, is key to the development and progression of bowel cancer.
Although the importance of epigenetics in cancer has long been realised, scientists have not known its specific mechanisms. Thanks to this latest research, they now understand how epigenetic control can affect bowel cancers, separately from the influence of DNA mutations. Both studies were co-led by Professor Trevor Graham, Director of the Centre for Evolution and Cancer, and Professor Andrea Sottoriva, Team Leader of the Evolutionary Genomics and Modelling team.
In the first study, the team used 1,373 samples from 30 bowel cancers to confirm that epigenetic changes are highly common in cancerous cells, are heritable and can bestow cancer cells with survival advantages. They also affect the accumulation of DNA mutations in the cancer cells. The second study focused on the DNA sequences of samples from different parts of the same tumour, showing that less than 2 per cent of changes in the DNA code in independent areas of a tumour are associated with changes in gene activity. Therefore, while DNA mutations are key for the development of cancer, epigenetics may determine much of how cancer cells behave.
Healthcare professionals currently test cancers for DNA mutations, but these tests do not reveal epigenetic changes, so they cannot always predict how a cancer will respond to treatment. The researchers believe that testing for both genetic and epigenetic changes in how genes are read is essential to optimise the treatment plan, and in turn the outlook, for each individual with cancer.
ICR-discovered drug to enter phase II clinical trial in ovarian cancer thanks to new research collaboration
May 2023

Image: The Centre for Cancer Drug Discovery building - now the home of our drug discovery scientists
In a phase I trial, a cancer drug discovered by scientists now based in the Centre for Cancer Drug Discovery at the ICR proved to be safe and effective enough to enter phase II trials. At this stage of development, idetrexed has shown comparable efficacy to drugs that have proceeded to late phase development.
Professor Udai Banerji, Deputy Director of the Drug Development Unit at the ICR, was Principal Investigator of the study, which enrolled 42 people for the dose escalation phase, with 67 people joining the trial once the optimal dose had been confirmed. All 67 of those in the expansion cohorts had high-grade serous ovarian cancer, which is the most common type of ovarian cancer. It typically goes undiagnosed until the later stages and commonly develops drug resistance, meaning that it often leads to poor outcomes.
Idetrexed has a unique mechanism of action. It works by targeting the alpha folate receptor (FRα) – which is expressed more highly in cancer cells than in noncancerous tissue – and then triggering cell death. In the trial, the recommended phase II dose of idetrexed led to tumour shrinkage in 36 per cent of the people with high or medium expression of FRα. The researchers are excited by the drug’s potential to save lives and look forward to seeing what it can achieve in the phase II trial. This trial was made possible by an agreement between Algok Bio and BTG International, which played a part in the drug’s development.
Discovery could lead to new drugs to block protein that fuels bowel cancer
November 2022

Image: Using cryo-electron microscopy, researchers obtained a detailed map (shown in orange) of the chain-like tankyrase structure. By interpreting this map and reconstructing the tankyrase molecules amino acid by amino acid (red), they deciphered how tankyrase is activated by ‘self-assembling’. Credit: Sebastian Guettler, ICR.
A team led by Professor Sebastian Guettler, Deputy Head of the Division of Structural Biology at the ICR, has gained crucial insights into a poorly understood protein that has a key role in the development of bowel cancer.
Scientists have long known that the tankyrase protein has links to cancer and that it supports Wnt signalling, which plays a part in cell division and development. They have even developed drugs to block tankyrase, but insufficient information about the protein means that these drugs currently cause too many side effects to enter clinical trials.
In a recent study, Professor Guettler and his colleagues used an extremely powerful type of microscopy that freezes samples at -180°C so that they could determine the protein shape down to the finest detail. They demonstrated that tankyrase self-assembles into fibres, changing its 3D structure to switch from inactive to active.
They propose that by designing structurally different tankyrase inhibitors that target specific regions of the protein – those that allow it to change its structure – it will be possible to treat bowel cancer more safely. Hyperactive Wnt signalling is a hallmark of almost all bowel cancers, so an effective targeted treatment could significantly improve the outcomes of thousands of people.
The team’s groundbreaking discovery could also have wider implications, as tankyrase helps regulate processes that contribute to multiple diseases, including diabetes and some inflammatory, cardiac and neurodegenerative diseases.
New study unveils epigenetic ‘traffic lights’ controlling stop and go for gene activity
March 2023

Image: Computer visualisation of DNA structure. Credit: Pixabay
A new study has transformed our understanding of the regulation of cell development and the process of gene expression. A team of researchers led by Professor Kristian Helin, Chief Executive of the ICR, was finally able to solve a decades-old mystery – how epigenetic proteins control the processes by which people’s genes are translated into proteins.
They found that H3K4me3, a chemical tag with a previously unknown role, acts as a key epigenetic signal, determining when and how DNA is read and translated. Enzymes place H3K4me3 on DNA, where it regulates the flow of RNA polymerase II, a protein complex that moves along DNA and transcribes it into RNA.
Without H3K4me3, RNA polymerase II stops moving, putting a halt to transcription. Previous research has linked the enzymes that place H3K4me3 on the DNA to various types of cancer, suggesting that drugs that target this chemical tag could be effective in defeating the disease.
Professor Helin believes that this work could go into textbooks, noting that fundamental scientific discoveries such as this are the foundation on which even the most cutting-edge treatments are built.
Scientists discover a new way to help prevent breast cancer ‘time bomb’
March 2023
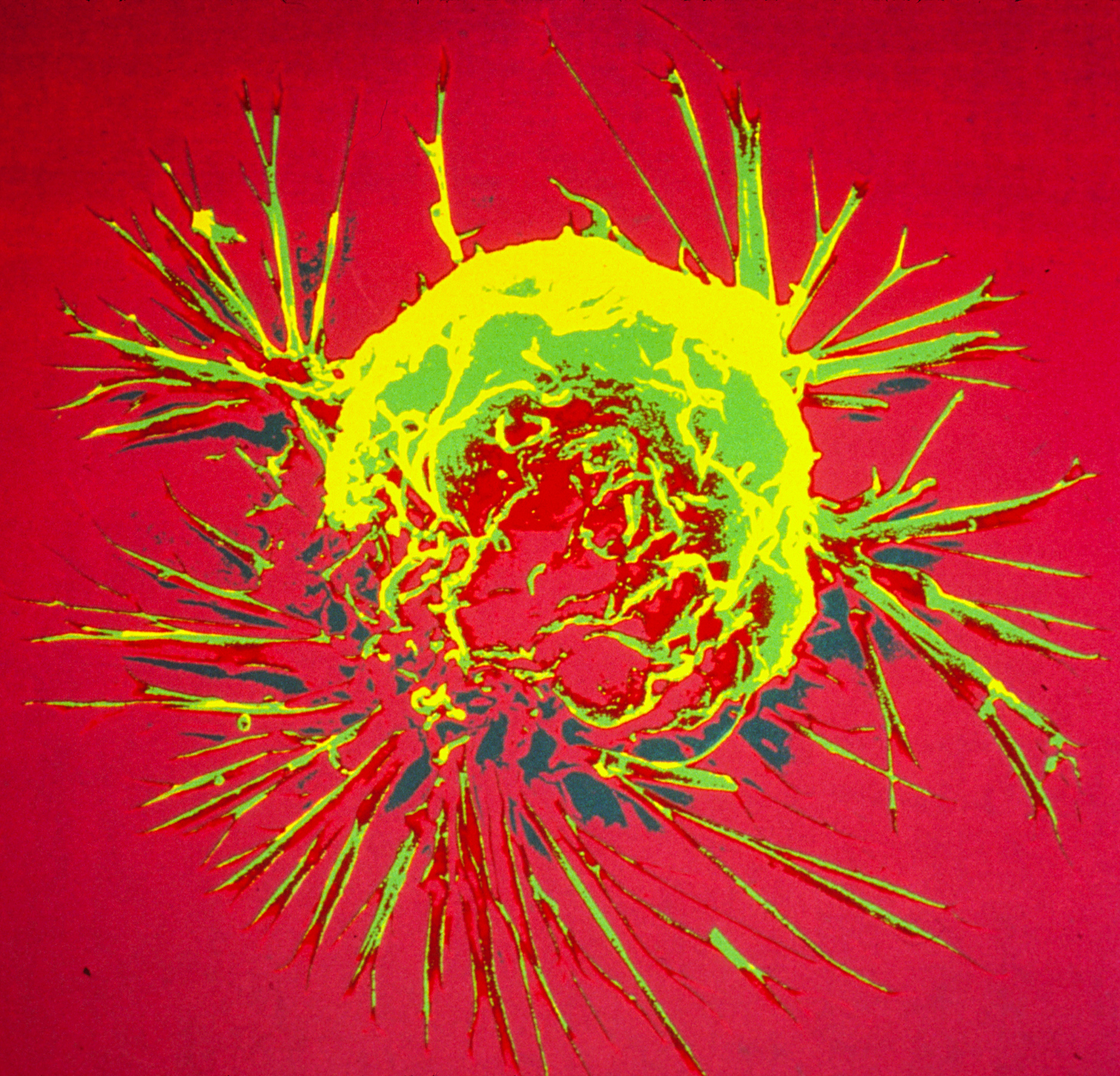
Image: Breast cancer cell spheroid, SEM. Credits: Khuloud T. Al-Jamal, David McCarthy, Izzat Suffian (CC BY 4.0)
Researchers in the Breast Cancer Now Toby Robins Research Centre at the ICR have discovered that age-related changes in the level of a certain protein in the lung influence the development of secondary tumours in people with the most common type of breast cancer.
After completing treatment, many people with oestrogen receptor positive (ER+) breast cancer experience a recurrence of the disease. This occurs due to breast cancer cells spreading to other parts of the body, where they form incurable secondary tumours. Although breast cancer cells that travel to the lungs may remain inactive for years or even decades, they can suddenly ‘wake up’.
The team, led by the ICR’s Dean of Academic and Research Affairs, Professor Clare Isacke, discovered that an increase in the level of PDGF-C protein in the lung can cause the dormant cells to grow. This increase typically occurs in lungs that are ageing or have sustained tissue damage. The researchers then demonstrated that a cancer growth blocker called imatinib could significantly reduce tumour growth in mice with ER+ tumours by targeting PDGF-C signalling.
About four in five cases of primary breast cancer are ER+, so future treatments that use this approach could benefit thousands of people by preventing the mechanism that causes dormant cancer cells to ’reawaken’.
Researchers gain fascinating new insights into chromosome shortening – and identify new potential cancer drug targets
August 2023
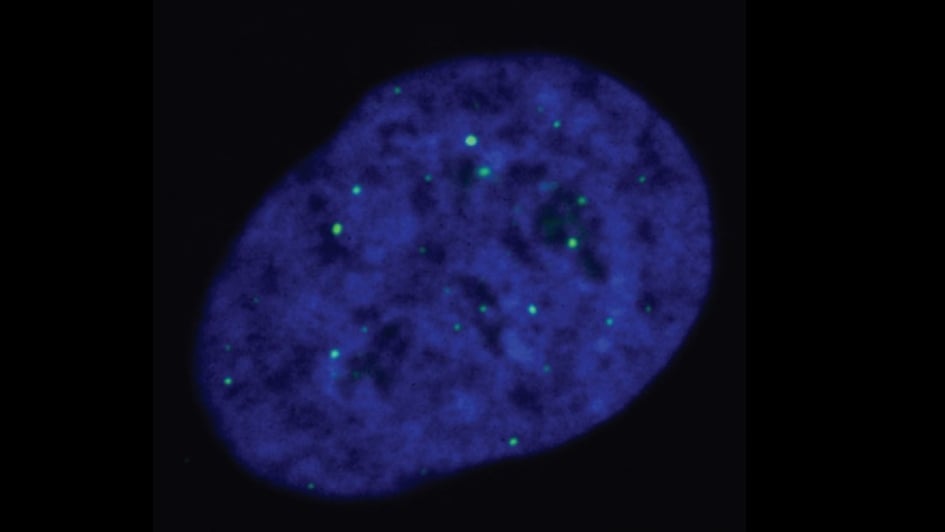
Image: ALT-reliant cells, with telomeres being marked by the green dots. Credits: Cancer and Genome Stability Team at the ICR
Innovative work in cells has identified a possible new druggable target for a subtype of cancers. Professor Wojciech Niedzwiedz, who leads the Genome Instability and Cancer Team at the ICR, co-led the study, which uncovered one of the mechanisms behind the survival of cancer cells.
The team showed that a protein called EXD2 nuclease has an important role in the ALT pathway, which supports survival in more than 10 per cent of cancers. ALT-reliant cancers use this pathway to avoid cell death, usually the inevitable result of multiple cell divisions. Specific DNA protein structures called telomeres sit at the end of chromosomes, and they shorten each time the cell divides. The ALT pathway helps cells counter telomere shortening via an unknown method, allowing them to maintain the length of their telomeres.
ALT-reliant cancers are highly aggressive, and the treatment options are very limited. Poor clinical outcomes are common, so there is a real need for effective treatments. In this study, the researchers found that EXD2 is necessary for telomere maintenance and that its absence leads to telomere shortening, making cell death the outcome in time. In addition, combining EXD2 depletion with the loss of other DNA repair proteins killed ALT-reliant cancer cells. They therefore believe that it could be possible to eradicate tumours relying on the ALT pathway for survival by targeting EXD2 nuclease.
Cell ‘nanobot’ breakthrough shines light on cause of aggressive cancers
May 2023
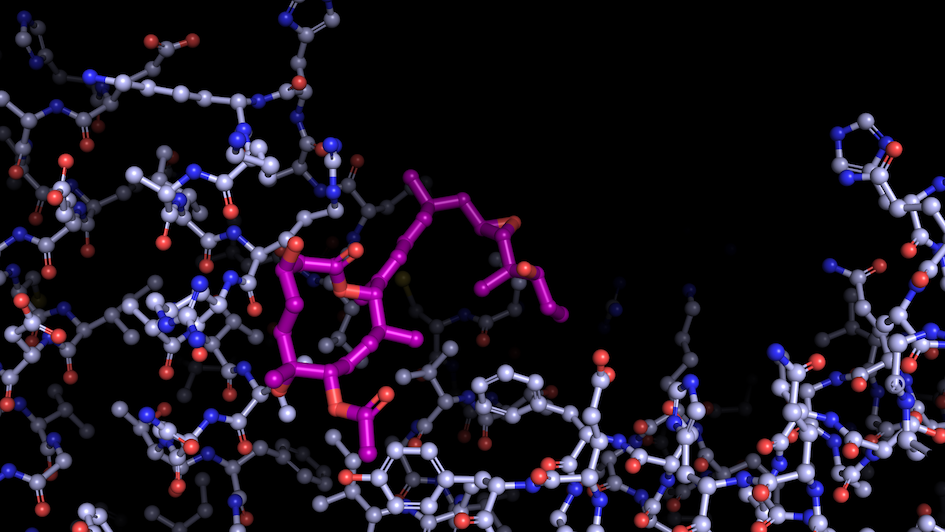
Image: “Like a constellation of atoms” – splicing modulator (purple) bound to a spliceosome subunit. Credit: Professor Vlad Pena
Scientists from the Structural Biology division at the ICR, led by Professor Vlad Pena, have uncovered a key mechanism behind the activation of RNA splicing, the process by which the non-coding sections of RNA are removed and the coding regions are joined.
Splicing is essential for gene expression, but it is often dysregulated in cancer. Mutations in the spliceosome – the cellular machinery responsible for splicing – can produce abnormal proteins that lead to cancer by promoting tumour growth or deactivating protective proteins.
Capturing the spliceosome mid-action for the first time allowed the researchers to determine that two molecular motors, called helicases, reshape a core spliceosome subunit called SF3B1. This change in shape causes splicing to recommence.
One of these molecular motors, PRP2, acts in a very unexpected way, travelling along the RNA strand being processed and rearranging the spliceosome as it goes. The researchers believe that other helicases may also use this technique, opening up potential new areas of research. This breakthrough finding should help scientists develop cancer drugs that target the splicing process, preventing the production of proteins that contribute to cancer.
Major study reveals how pancreatic cancer changes its ‘diet’ to survive
May 2023
Image: Pancreatic cancer cells. Credit: Anne Weston, Francis Crick Institute (CC BY-NC 4.0)
Dr Anguraj Sadanandam, Team Leader in Systems and Precision Cancer Medicine, co-led a study that has opened the door to a new treatment approach for pancreatic ductal adenocarcinoma, the most common and aggressive form of pancreatic cancer.
Using a phenotypic microarray, which makes it possible to monitor the reaction of different cells to environmental challenges or introduced compounds, the researchers tracked which nutrients the pancreatic cancer cells used over three days. They found that when these cells do not have access to their usual food source, glucose, they can use a molecule called uridine instead.
Uridine supports a healthy metabolism in humans, and it is available around the body. It is broken down by the uridine phosphorylase-1 (UPP1) enzyme to form a type of sugar called ribose, which provides cancer cells with energy. When they looked at patient samples, the researchers noted a correlation between high levels of the UPP1 gene and poor survival in people with pancreatic cancer.
The team then showed that blocking the UPP1 gene in mice with pancreatic cancer halted most tumour growth by preventing the cancer cells from breaking down uridine. The next step is to develop treatment strategies that target uridine availability to help extend and improve the lives of people with pancreatic cancer.
New targeted drug shows benefit against breast cancer in first phase III trial
December 2022

Image: False coloured scanning electron micrograph of a breast cancer cell from a cultured cell line. Credit: Anne Weston, Francis Crick Institute (CC BY-NC 4.0)
The first phase III trial to report findings on a new type of targeted medicine has produced remarkable results. A combination of the drug capivasertib and hormone therapy was effective for all 708 people in the international CAPItello-291 trial, many of whom had seen their cancer recur or progress following standard hormone treatments.
Outside of clinical trials, chemotherapy is currently the only effective option for these patients, but it can cause debilitating side effects. The trial was led by Professor Nicholas Turner, Professor of Molecular Oncology at the ICR and both Consultant Medical Oncologist and Head of the Ralph Lauren Centre for Breast Cancer Research at The Royal Marsden.
Capivasertib works by blocking the activity of a protein kinase called AKT, which stimulates cell growth. Including capivasertib in the treatment doubled the time it took for the participants’ cancer to progress, extending the median time to disease progression from 3.6 months to 7.2 months. In addition, the combined treatment led to tumour shrinkage in 23 per cent of the participants, compared with 12 per cent of those receiving hormone treatment plus a placebo.
All of the participants had locally advanced or metastatic breast cancer that was positive for oestrogen receptor (ER+) and low or negative for human epidermal growth factor receptor 2 (HER2). However, the treatment was most effective for the 41 per cent of people whose cancers also had genetic alterations to the AKT signalling pathway. These alterations can promote cancer and increase treatment resistance. Among these patients, those who received the combination treatment had a median progression-free survival of 7.3 months while 29 per cent saw their tumours shrink.
These results are particularly exciting because ICR research was fundamental to the discovery of capivasertib. The team behind the CAPItello-291 study is hopeful that capivasertib combined with hormone therapy will become a new treatment option for people with certain types of breast cancer.
Radiotherapy boost cuts breast cancer treatment time by at least one week
July 2023
---945x532.jpg)
Image: Intensity modulated radiotherapy (IMRT). Credit: Jan Chlebik/the ICR
A phase III trial managed by the ICR’s Clinical Trials and Statistics Unit (ICR-CTSU), led by Professor Judith Bliss, demonstrated that simultaneous integrated boost (SIB) radiotherapy reduces the duration of treatment for patients with early stage breast cancer by at least a week. This approach, which involves giving patients a targeted additional dose of radiotherapy at the same time as treatment to the whole breast, also proved to be just as effective as existing radiotherapy techniques in lowering the risk of recurrence.
A total of 2,617 patients took part in the IMPORT HIGH trial, where they underwent one of three treatment regimens. All three groups received whole breast radiotherapy and a targeted boost. However, one group received the boost after the whole breast radiotherapy – known as a sequential boost – whereas the other two groups received simultaneous boosts at different doses. Delivering the boost simultaneously only increased the rate of side effects at the higher dose.
In 2020, results from another ICR-CTSU- led study, FAST-Forward, revealed that it was possible to deliver whole breast radiotherapy over the course of one week. Researchers in the unit now hope to run another clinical trial to determine whether this timeline is also possible using SIB radiotherapy.
Reducing the duration of treatment benefits not only patients, who have to make fewer hospital visits, but also cancer services, which are dealing with chronic staff shortages and struggling to prevent waiting lists from becoming overly long.