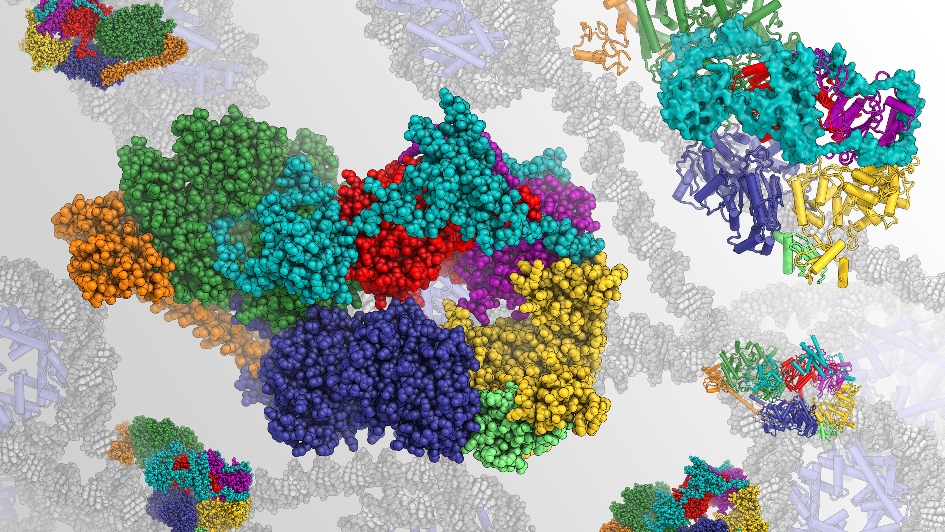
Image: Rendering of the structure of human transcription factor IIH, a focus of Dr Basil Greber's research. This molecular complex is involved in transcription and repair of DNA. Credit: Dr Basil Greber
This week, schools and institutions across the country are celebrating Biology Week 2020, a time where we hope to inspire others and open up conversations about biology. Here at the Institute of Cancer Research, biology takes centre stage in a lot of our research – from looking at how cells and genes function down to studying tiny molecules that keep us alive.
Those tiny molecules, called proteins, are what Dr Basil Greber takes particular interest in. As a structural biologist, he looks at how some of those proteins are built to try and understand what happens when they malfunction and people get cancer. Dr Greber recently joined the ICR’s Division of Structural Biology as a team leader, so I spoke to him to find out more.
“Proteins are like tiny machines”
“We are trying to find out how protein molecules inside our bodies do their jobs,” he tells me. To explain the method, he describes the proteins as tiny machines that perform all kinds of functions inside our cells to keep us alive – and how studying those tiny machines is a bit like figuring out how a bigger machine, such as a car engine, works.
There’s two ways you could go about understanding how a car engine works, he explains – you could either fill the tank with fuel and measure what comes out of the exhaust pipe to see what happens to the fuel inside the engine, or you could open the hood and look at each individual part to understand their function and why they’re always arranged in a specific way.
“As structural biologists, we primarily do the latter, except that we cannot just open the hood and look. Because the molecules we study are tiny, on the order of a millionth of a centimetre long or so, we need to use extremely advanced technologies, such as electron microscopy, to see them. But once we see them in sufficient detail, we can figure out how they do the things they do, and sometimes we can also understand what happens when they malfunction,” Dr Greber explains.
“Resolution revolution”
The technology Dr Greber uses to find out the structure and function of particular proteins is called cryo-electron microscopy (or cryo-EM). What’s special about this method is that the samples are flash-frozen so rapidly that the solution containing the proteins does not even have time to form ice crystals while freezing, forming ‘vitreous’ ice that is ‘transparent’ to electrons, enabling a picture to be formed without being impeded by crystals. This rapid freezing also means the molecules remain in a very similar state to how they would appear in our cells.
When Dr Greber first started to work on understanding the structure of particular proteins, during his MSc thesis work at the ETH Zurich in Switzerland, the technologies available to him could achieve only low-resolution reconstructions of the molecules that he studied. But Basil experienced a complete revolution in his field:
“Cryo-electron microscopy, one of the primary methods that I use, has experienced such dramatic development in the last seven or eight years that it completely changed the field. We call it half-jokingly, half-seriously, the ‘resolution revolution’”.
Researchers at the ICR have pioneered the use of cryo-EM in cancer research and were one of the founding members of LonCEM, a new consortium which is pooling knowledge from across the academic sector in London and recently purchased a new state-of-the-art microscope, thanks to a £3m grant from Wellcome.
It’s an exciting time for the field, as Dr Greber says.
“Today, our cameras are extremely fast and very efficient. As a result, the very best 3D-reconstructions generated by cryo-EM now can literally resolve individual atoms”, he explains.
Understanding the structure of a DNA repair mechanism
The molecules Dr Greber focuses his primary efforts on are involved in the repair of damaged DNA, which stores the genetic information of our cells such as the information needed to build proteins.
It’s crucial that the information stored in our DNA stays intact – “otherwise, the cells in our bodies may get wrong instructions and die, or they may get wrong instructions and start acting up – growing too much, dividing too rapidly, and accumulate even more damage to their DNA, ultimately turning them into cancer cells,” he explains.
The molecules that Basil works on are part of a mechanism called nucleotide excision repair that detects damage in our DNA and fixes it. The mechanism plays a crucial role – if it doesn’t operate properly, it could result in human disease, including cancer.
Figuring out the structures of the proteins involved will help us understand exactly how this mechanism works, and where it can go wrong. He explains:
“Nucleotide excision repair is an intricate and highly coordinated process. We want to image all the different molecular structures along that pathway to obtain a ‘molecular movie’ of the process.”
Dr Greber also collaborates with Professor Wojciech Niedzwiedz at the ICR and other teams in London to put his findings into a wider context in order to understand how exactly the whole mechanism works, based on the structures he identifies.
A place nobody has ever been to before
When I asked him what his favourite part of his job is, he replied:
“Seeing the 3D-reconstruction of a large molecular complex for the first time is probably my favourite part. It means to see something nobody else may have seen before, and we can spend weeks or months exploring it on our computer screens, like a three-dimensional map of a place nobody has ever been to before.”
Having only just started, Dr Greber does not have a team yet – but looks forward to mentoring graduate students, building a new lab, and charting his own path in science.
comments powered by