DOCK guanine nucleotide exchange factors (GEFs)
DOCK proteins have been implicated in the activation of Rac and Cdc42 in cell migration, morphogenesis and phagocytosis, and as important components of tumour cell movement and invasion.
In humans, DOCK proteins are organised into four subfamilies, characterised by their differing specificities for Rac and Cdc42, and associated regulatory domains and subunits. DOCK A and B subfamilies activate Rac, the DOCK D subfamily is specific for Cdc42, whereas the DOCK C subfamily has dual specificity for Rac and Cdc42. All contain a GEF catalytic domain (DHR2) of ~400 residues situated within the C-terminus and a second conserved region, the ~250 residue DHR1 domain.
Our initial efforts were focussed on the DHR2 domain of DOCK9 (DOCK9DHR2), a Cdc42-specific GEF and we generated numerous constructs of DOCK9DHR2 to identify the N-terminus corresponding to a functional and structural domain. A complex of DOCK9DHR2 with Cdc42 was crystallised, and we determined structures of the holoenzyme and complexes with GDP and GTP.
Comparison of these structures revealed the full catalytic mechanism by which DOCK proteins mediate the release of GDP from the GTPase, and then how the subsequent binding of GTP stimulates the discharge of the activated GTPase from the exchange factor (Yang et al., 2009). This work also demonstrated that all DOCK proteins dimerise through their DHR2 domain.
More recently we have applied a similar approach used to generate DOCK9DHR2-Cdc42 complexes to generate DOCK2DHR2-Rac1 and DOCK10DHR2-Cdc42 complexes. DOCK2 is highly related to DOCK1/180, a member of the family implicated in oncogenesis (as is DOCK10). DOCK2 is a Rac specific DOCK protein. We have crystallised DOCK2DHR2-Rac1 and DOCK10DHR2-Cdc42 and have determined their three-dimensional structures. We have used site directed mutagenesis to test our structural predictions for the molecular basis for DOCK2 specificity for Rac1 and DOCK9/10 specificity for Cdc42.
Our future work will focus on the full-length DOCK proteins (DOCK1 to 5 form complexes with an adaptor protein ELMO). We will express DOCK9 and DOCK10 and DOCK1-ELMO and DOCK2-ELMO using the insect cell system and analyse their structures using single particle EM analysis. Their large size (dimers and heterotetramers) will facilitate EM analysis.
Due to their role in metastasis, DOCK proteins are valid anti-cancer drug targets, consequently, we are developing methods to inhibit DOCK GEF catalytic activity.
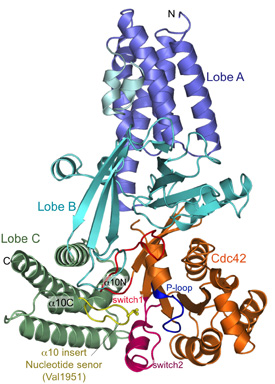
Above: Views of nucleotide free DOCK9DHR2-Cdc42. (A) Overall structure. Lobes A, B, and C colored in blue, cyan, and green, respectively; alpha-10 insert with Val1951 (nucleotide sensor) in yellow. Cdc42 is coloured orange with switch 1 and 2 and P loop in red, purple, and blue, respectively. From Yang et al., (2009).