
Image: Professor Ian Collins discusses designing the cancer drugs of the future with members of the Discovery Club
Members of the ICR’s Discovery Club saw first-hand the delicacy and complexity of techniques used to sculpt new drug molecules, as they learned how our scientists design the cancer drugs of the future.
At an evening at The Royal Society of Chemistry, Professor Ian Collins and Dr Rob van Montfort described the cutting-edge chemistry and structural biology they use to build compounds on the scale of individual atoms.
Alongside colleagues, they demonstrated how chemical reactions are used to construct molecules and how powerful X-rays are used to understand their shape. Sophisticated modelling also showed how molecules bind with cancer-causing proteins to build better drugs.
The event was a fascinating insight into the world-class drug discovery research taking place at The Institute of Cancer Research, London, which is transforming the lives of patients with cancer.
Molecular architects
The Discovery Club heard from two of our molecular architects, Professor Collins and Dr van Montfort, who work together using their expertise in medicinal chemistry and structural biology to build the cancer drugs of the future.
Professor Collins described how our scientists are helping to identify the folds and crevices on proteins that are important in cancer and designing small molecules to fit.
By understanding the atomic structure of the proteins, researchers can design and synthesise drugs that fit perfectly into their target site, knocking out the protein’s activity.
Triple negative breast cancer is an aggressive form of breast cancer that currently has limited treatment options. Professor Collins talked about a new cancer drug giving new hope for those patients.
He described the story of the discovery and development of capivasertib, a targeted drug which has recently shown potential benefit in clinical trials for the disease.
The drug, formerly called AZD5363, was discovered following a collaboration including ICR researchers and was developed through an early stage trial led by the ICR and its partner hospital The Royal Marsden NHS Foundation Trust.
Professor Collins said:
“For a lot of the drugs we’re trying to make, this will be the first time this molecule has ever existed anywhere.
“As a chemist that’s what really excites me, to hold in your hands something that is probably the first time in the universe this particular arrangement of atoms has ever been created. That’s fantastic and it is the creative discipline of synthetic chemistry.”
Demonstrating the drug discoverer’s toolkit
Dr van Montfort (image below) told the Discovery Club how his team study crystallised proteins to understand their shape in order to build drugs that bind tightly.

His team work with powerful X-ray microscopes, which are needed to see proteins on the atomic scale.
Through a painstaking process of building molecules and seeing how they bind to their target protein, researchers can hone in on those that are the most potent and have the greatest potential to make a good cancer drug.
Scientists from Professor Collins and Dr van Montfort’s team (image below) showed the Discovery Club the cutting-edge techniques involved in their work. They demonstrated the tools they use to understand the structures of proteins and build cancer drugs.

The Discovery Club provides its members with personal access to the science behind our discoveries. Through special events and updates, it is an opportunity to speak directly with the people who are working towards better outcomes for cancer patients and learn more about our research.
Find out more
Revolutionising cancer treatment
Professor Collins and Dr van Montfort will be working in our new Centre for Cancer Drug Discovery, due to open later this year.
The pioneering research programme within the building will focus on understanding, anticipating and overcoming the disease’s lethal ability to evolve and develop resistance.
Dr van Montfort said: “I hope that what Ian and I have shown is that you need skilled multidisciplinary teams to design cancer drugs, you can’t do it on your own.
“It takes a team science approach and the new Centre for Cancer Drug Discovery will allow us to work in a more integrated way, helping us work faster and more efficiently.”
We are now poised to outsmart cancer with the world’s first 'Darwinian' drug discovery programme. We believe this anti-evolution approach will deliver new and better treatments for cancer patients – ensuring even advanced disease can be managed long term and more often cured
Deputy Director of Philanthropy Hannah Joyce said:
“It was a fantastic event and it’s great that more people than ever are attending and learning first-hand about our world class drug discovery research.
“We had some fantastic feedback from our guests, particularly around the demonstrations from our young scientists, giving our Discovery Club members a glimpse into what it takes to develop new cancer drugs.”
Your support is vital to our work
The Discovery Club is our high-level giving club whose members help us to drive forward our scientific strategy through philanthropic investment in key organisational priorities.
The events are an opportunity for members to hear from leading ICR scientists and clinicians about their work, and the ways that their support is making a difference.
If you would like to find out more, please contact Hannah Joyce, Deputy Director of Philanthropy.
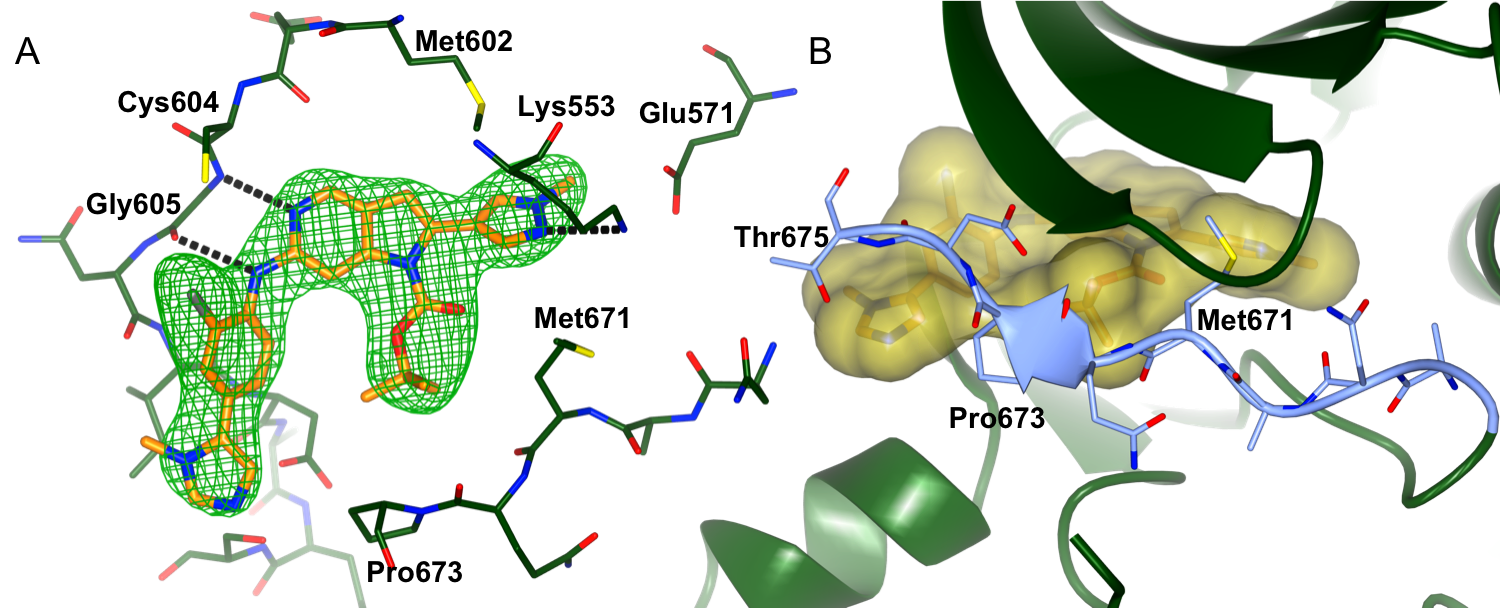 Figure 1: Binding of CCT251455 to MPS1. (A) Binding of CCT251455 to the hinge region of MPS1. (B) CCT251455 stabilises and unusual conformation of the activation loop (light blue).
Figure 1: Binding of CCT251455 to MPS1. (A) Binding of CCT251455 to the hinge region of MPS1. (B) CCT251455 stabilises and unusual conformation of the activation loop (light blue).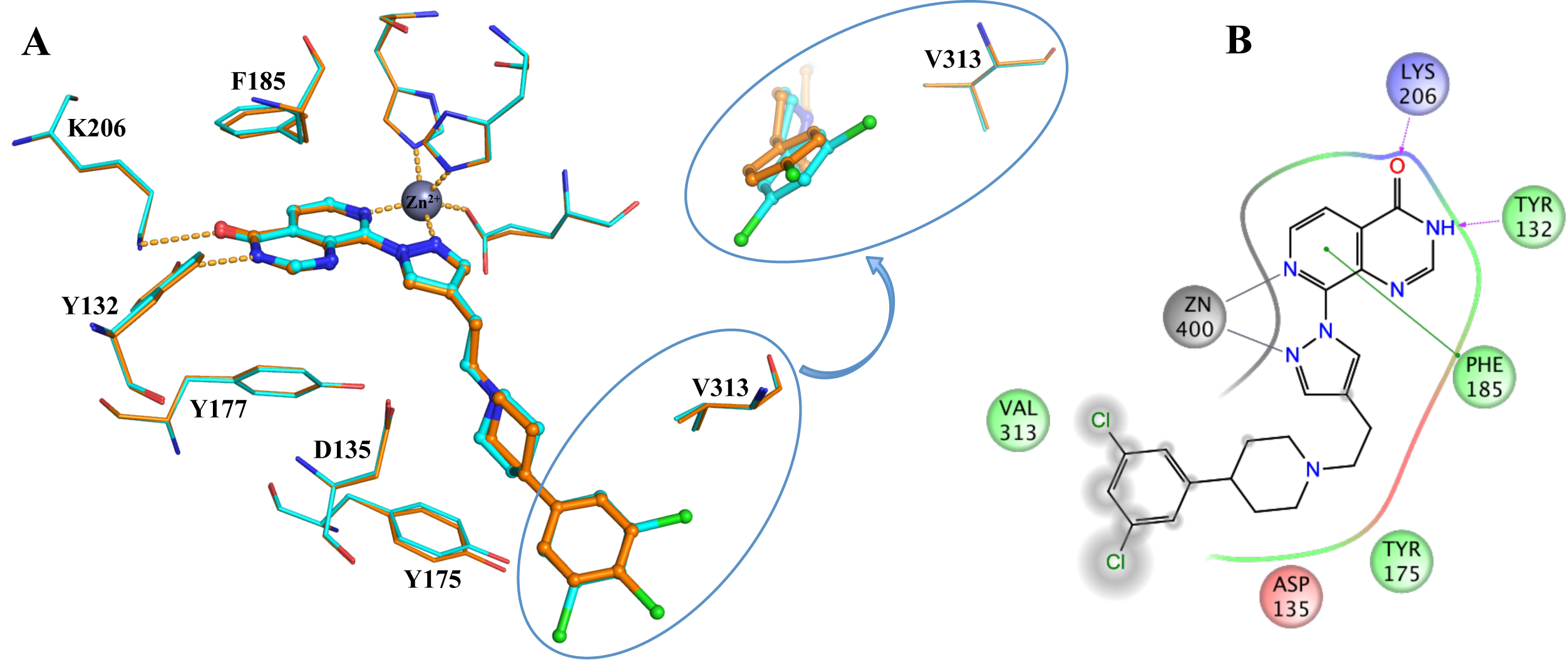 Figure 2: Binding of KDM4/5 inhibitors to KDM4A. A) Superposition of two KDM4/5 inhibitors (blue/organge) bound to KDM4A highlighting key interactions with the protein. B) 2D interaction diagram showing the interactions of the most potent inhibitor (orange) with KDM4A.
Figure 2: Binding of KDM4/5 inhibitors to KDM4A. A) Superposition of two KDM4/5 inhibitors (blue/organge) bound to KDM4A highlighting key interactions with the protein. B) 2D interaction diagram showing the interactions of the most potent inhibitor (orange) with KDM4A.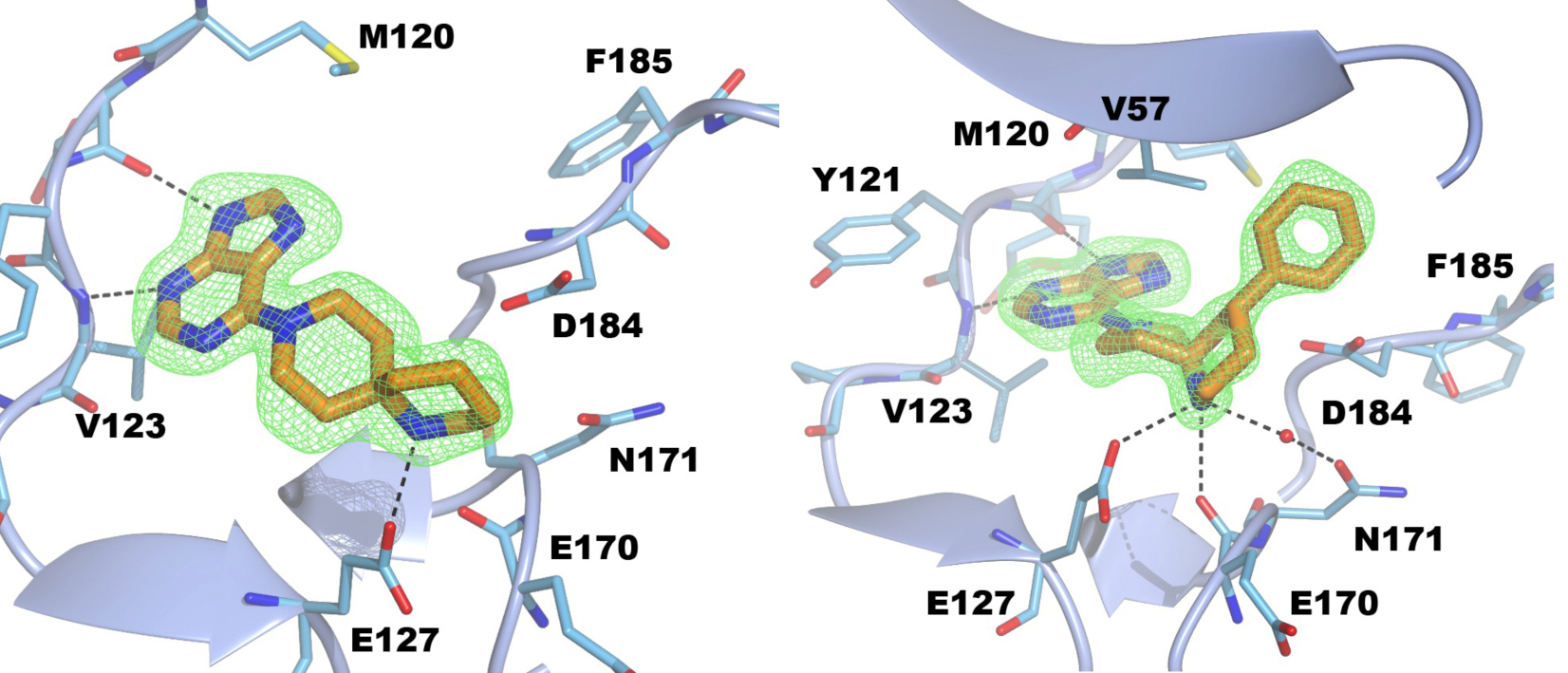
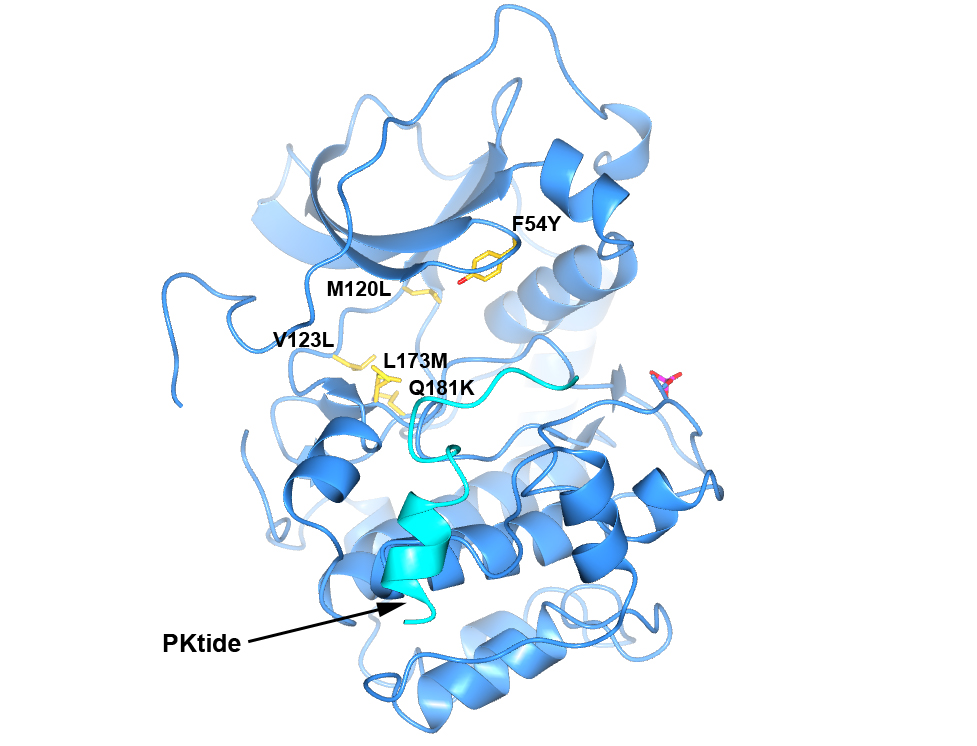 Figure 4. Structure of PKA with the five mutations in and near the ATP-binding site highlighted in yellow. The PKtide substrate inhibitor is shown in light blue
Figure 4. Structure of PKA with the five mutations in and near the ATP-binding site highlighted in yellow. The PKtide substrate inhibitor is shown in light blue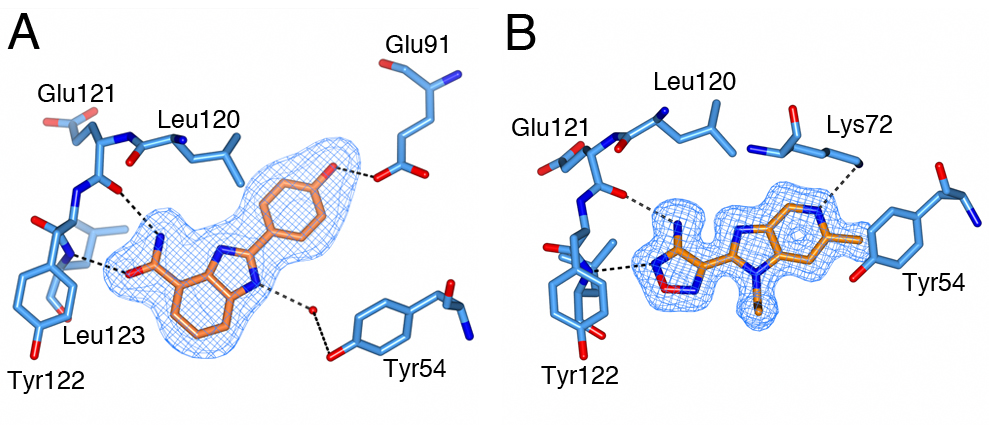 Figure 5. S6K inhibitors bound in the PKA-S6K1 chimeara. A) High resolution structure (2.2Å) of a carboxamidobenzimidazole HTS hit bound in the PKA-S6K1 ATP binding site. B) High resolution structure (1.6Å) of a benzimidazole oxadiazole S6K inhibitor bound in the PKA-S6K1 ATP binding site.
Figure 5. S6K inhibitors bound in the PKA-S6K1 chimeara. A) High resolution structure (2.2Å) of a carboxamidobenzimidazole HTS hit bound in the PKA-S6K1 ATP binding site. B) High resolution structure (1.6Å) of a benzimidazole oxadiazole S6K inhibitor bound in the PKA-S6K1 ATP binding site.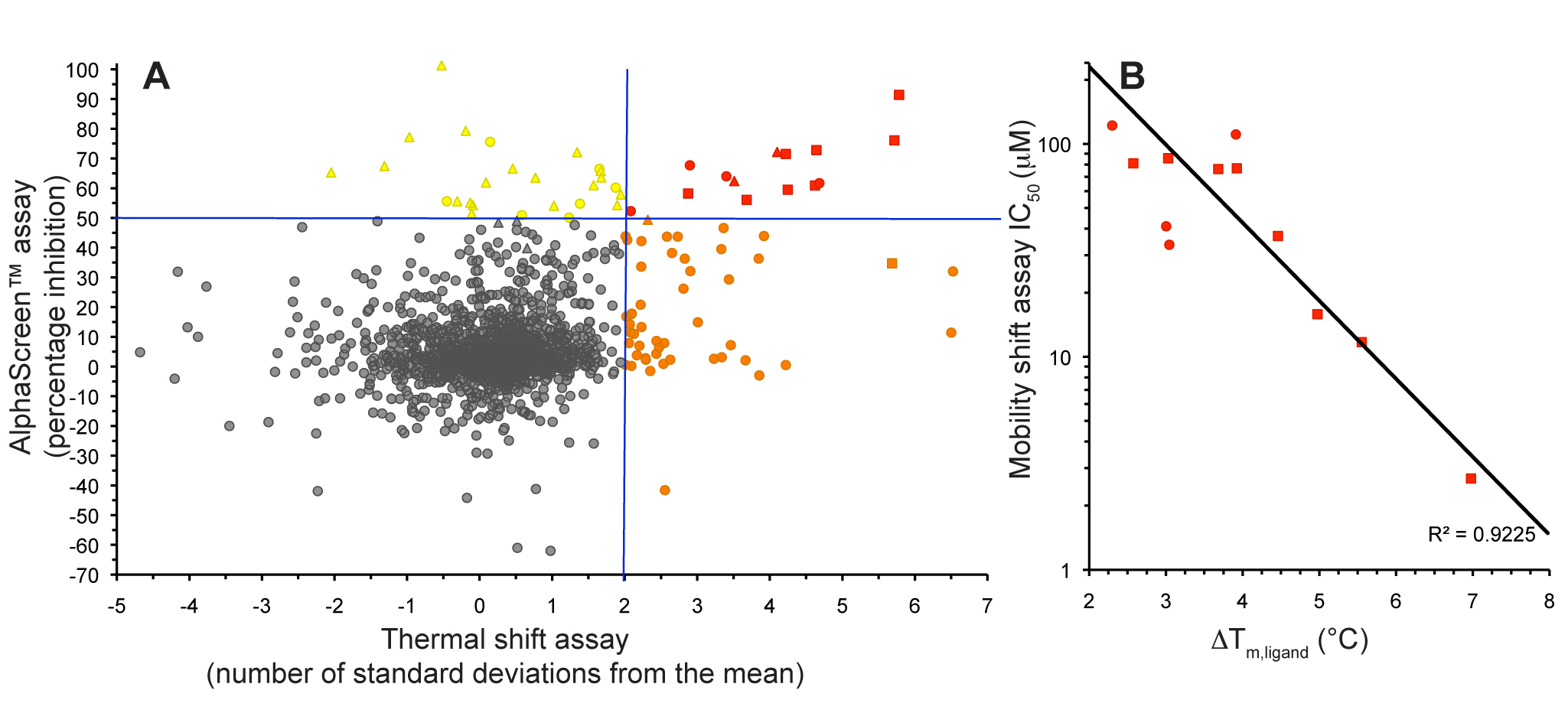 Figure 1. Fragment screening data from biochemical and thermal shift assays. (A) Comparison showing the primary AlphaScreen™ data plotted along the vertical axis as percentage inhibition, and the thermal shift data plotted along the horizontal axis as the number of standard deviations from the mean Tm, ligand for each plate and thresholds displayed as blue lines Hits in AlphaScreen™ and thermal shift are displayed in yellow and orange respectively. Mutual hits are shown in red, inactive fragments are grey. (B) Comparison of the IC50 and DTm, ligand values for the screening hits. Fragments showing interference in the AlphaScreen™ are indicated as triangles. Fragments confirmed in crystallography are shown as squares.
Figure 1. Fragment screening data from biochemical and thermal shift assays. (A) Comparison showing the primary AlphaScreen™ data plotted along the vertical axis as percentage inhibition, and the thermal shift data plotted along the horizontal axis as the number of standard deviations from the mean Tm, ligand for each plate and thresholds displayed as blue lines Hits in AlphaScreen™ and thermal shift are displayed in yellow and orange respectively. Mutual hits are shown in red, inactive fragments are grey. (B) Comparison of the IC50 and DTm, ligand values for the screening hits. Fragments showing interference in the AlphaScreen™ are indicated as triangles. Fragments confirmed in crystallography are shown as squares.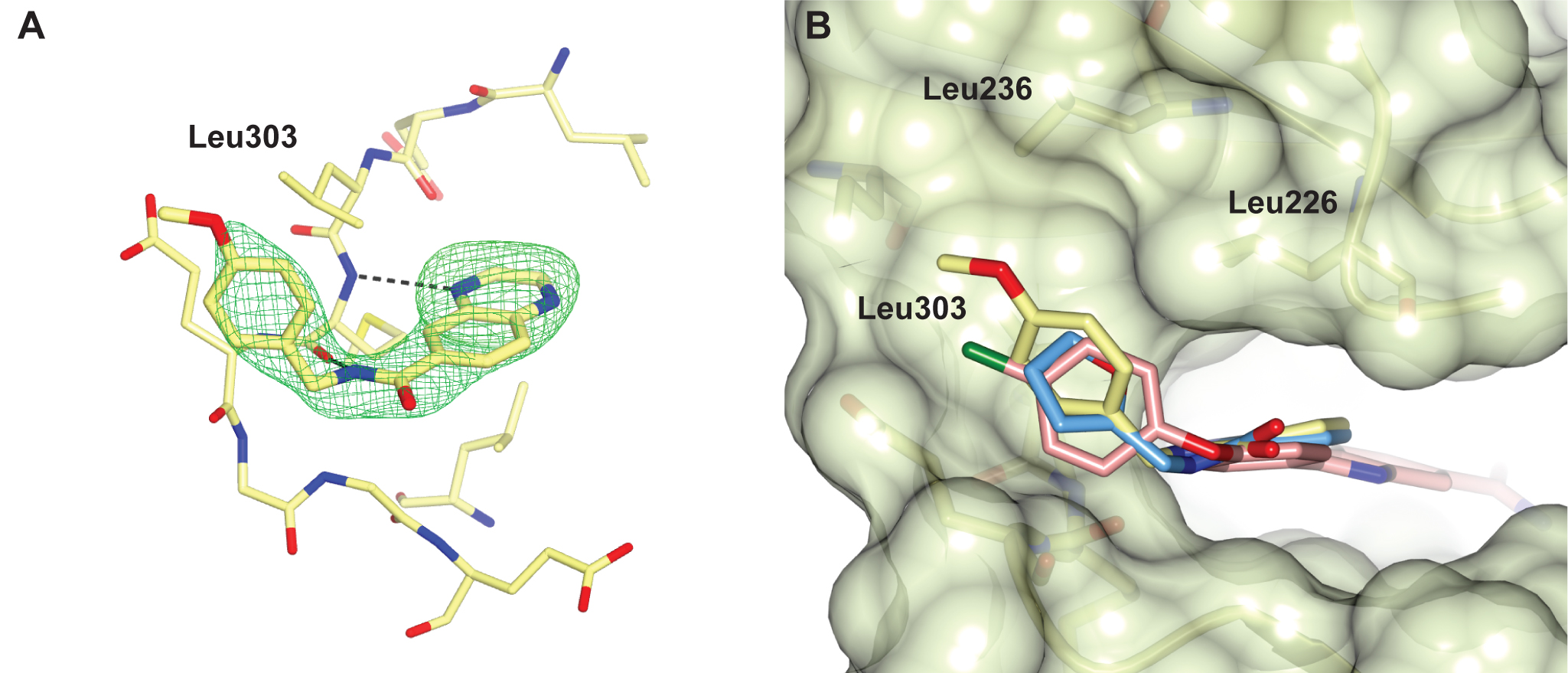
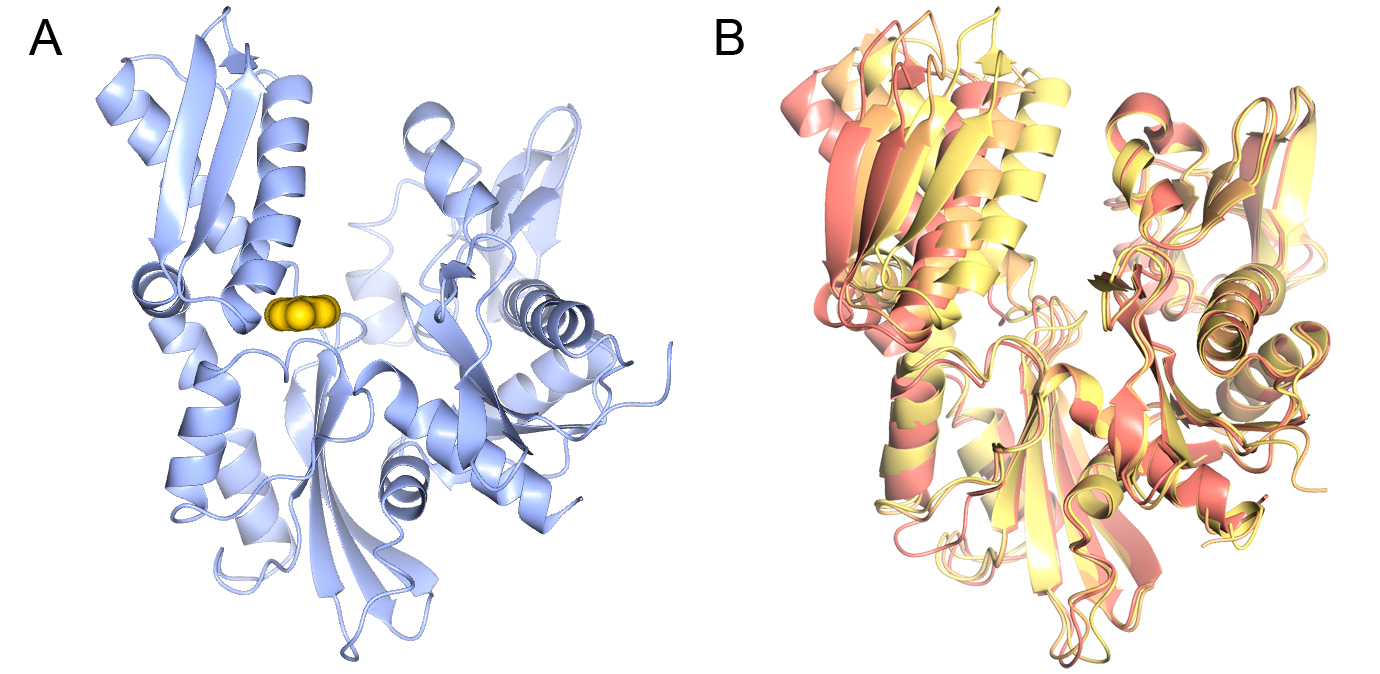
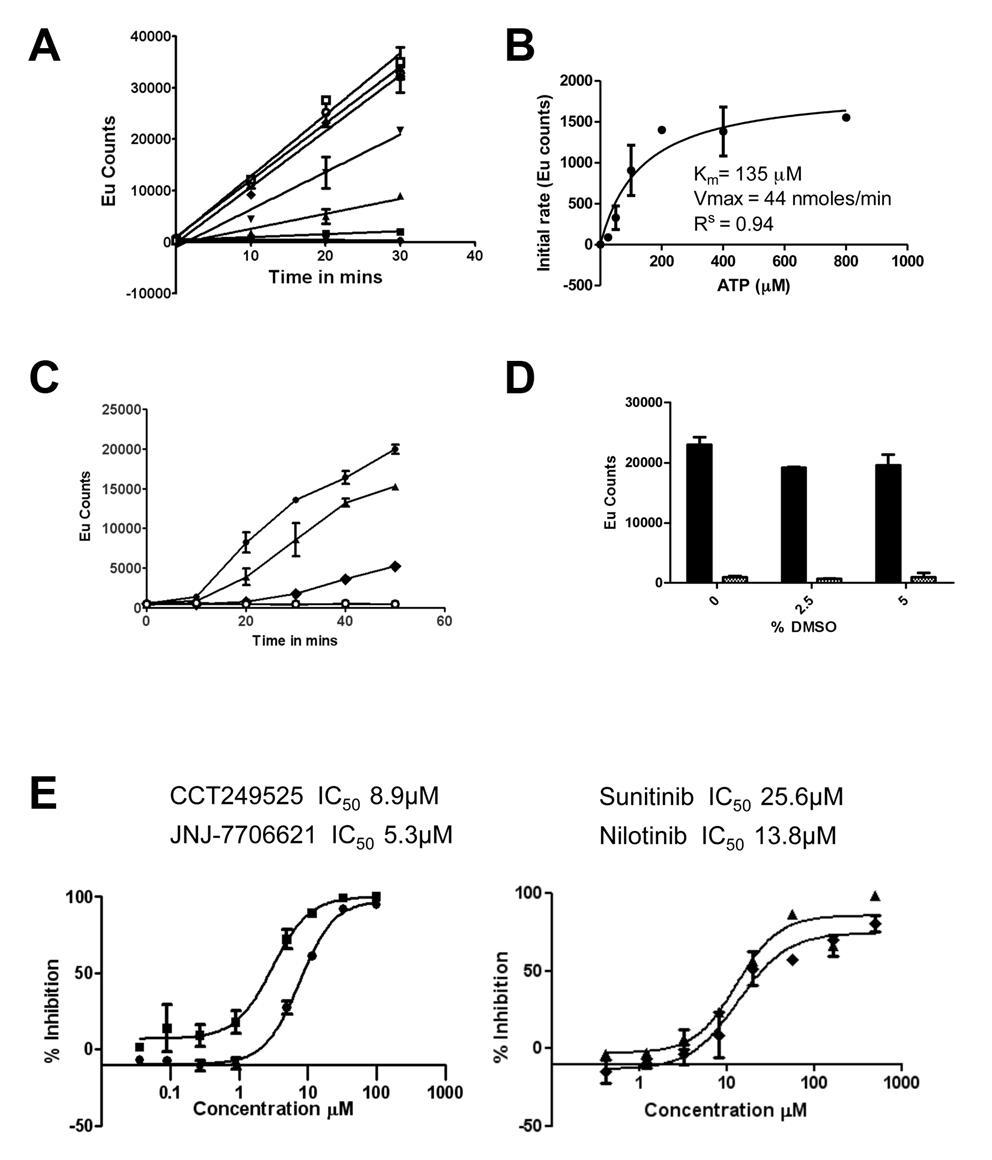 Figure 1. The IRE1α autophosphorylation DELFIA® assay. (A) Time course with varying ATP concentrations used to calculate the Km for adenosine triphosphate (ATP) (● no ATP, ■ 25 µM ATP, ▲ 50 µM ATP, ▼100 µM ATP,♦ 200 µM ATP, ○ 400 µM ATP,□ 800 µM ATP). B) Determination of the Km for adenosine triphosphate (ATP). C) Enzyme reaction time course; mean +/- SD of n=2 wells (● enzyme reaction at 700 nM IRE1α and 100 µM ATP, ▲ enzyme reaction at 500 nM IRE1α and 100 µM ATP, ♦ enzyme reaction at 350 nM IRE1α and 100 µM ATP,○). Enzyme reaction at 700 nM IRE1α without ATP). D) Effect of DMSO on the IRE1α autophosphorylation DELFIA®, showing no effect on the assay with up to 5% DMSO. E) Inhibition of IRE1α autophosphorylation activity by CCT249525 (●) , JNJ-7706612 (■), Sunitinib (♦) and Nilotinib (▲).
Figure 1. The IRE1α autophosphorylation DELFIA® assay. (A) Time course with varying ATP concentrations used to calculate the Km for adenosine triphosphate (ATP) (● no ATP, ■ 25 µM ATP, ▲ 50 µM ATP, ▼100 µM ATP,♦ 200 µM ATP, ○ 400 µM ATP,□ 800 µM ATP). B) Determination of the Km for adenosine triphosphate (ATP). C) Enzyme reaction time course; mean +/- SD of n=2 wells (● enzyme reaction at 700 nM IRE1α and 100 µM ATP, ▲ enzyme reaction at 500 nM IRE1α and 100 µM ATP, ♦ enzyme reaction at 350 nM IRE1α and 100 µM ATP,○). Enzyme reaction at 700 nM IRE1α without ATP). D) Effect of DMSO on the IRE1α autophosphorylation DELFIA®, showing no effect on the assay with up to 5% DMSO. E) Inhibition of IRE1α autophosphorylation activity by CCT249525 (●) , JNJ-7706612 (■), Sunitinib (♦) and Nilotinib (▲).
 .
.
 .
.