Paediatric Solid Tumour Biology and Therapeutics Group
Professor Louis Chesler’s group is investigating the genetic causes for the childhood cancers, neuroblastoma, medulloblastoma and rhabdomyosarcoma.
Research, projects and publications in this group
Our group's aim is to improve the treatment and survival of children with neuroblastoma, medulloblastoma and rhabdomyosarcoma.
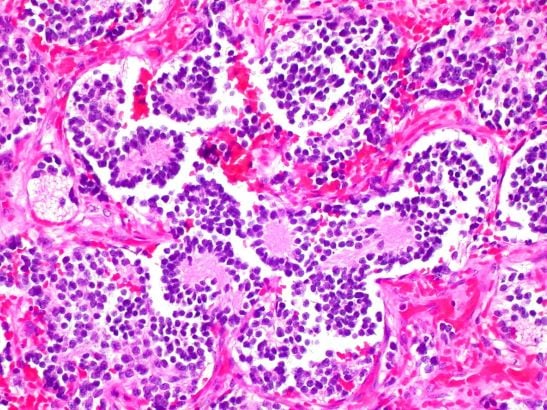
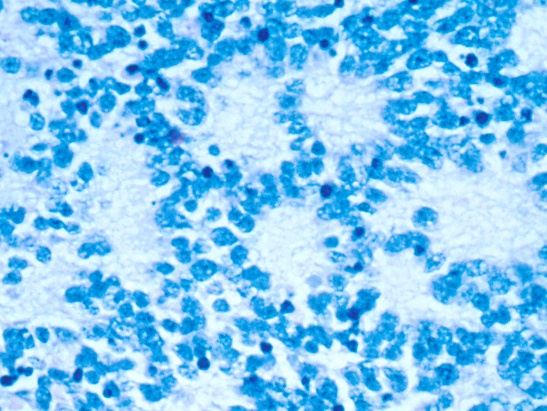
The goal of our laboratory is to improve the treatment and survival of children with neuroblastoma, medulloblastoma and rhabdomyosarcoma, three paediatric solid tumours in which high-risk patient cohorts can be defined by alterations in a single oncogene. We focus on the role of the MYCN oncogene, since aberrant expression of MYCNis very significantly associated with high-risk in all three diseases and implies that they may have a common cell-of-origin.
Elucidating the molecular signalling pathways that control expression of the MYCN oncoprotein and targeting these pathways with novel therapeutics is a major goal of the laboratory. We use a variety of innovative preclinical drug development platforms for this purpose.
Technologically, we focus on genetically engineered cancer models incorporating novel imaging (optical and fluorescent) modalities that can be used as markers to monitor disease progression and therapeutic response.
Our group has several key objectives:
- Mechanistically dissect the role of the MYCN oncogene, and other key oncogenic driver genes in poor-outcome paediatric solid tumours (neuroblastoma, medulloblastoma, rhabdomyosarcoma).
- Develop novel therapeutics targeting MYCN oncoproteins and other key oncogenic drivers
- Develop improved genetic cancer models dually useful for studies of oncogenesis and preclinical development of novel therapeutics.
- Use such models to develop and functionally validate optical imaging modalities useful as surrogate markers of tumour progression in paediatric cancer.
Professor Louis Chesler
Clinical Senior Lecturer/Group Leader:
Paediatric Solid Tumour Biology and Therapeutics.tmb-propic-md.jpg?Culture=en&sfvrsn=c25d2b2f_9)
Professor Louis Chesler is working to understand the biology of children’s cancers and use that information to discover and develop new personalised approaches to cancer treatment. His work focuses on improving the understanding of the role of the MYCN oncogene.
Researchers in this group
 .
.
I obtained an MSci in Biochemistry from the University of Glasgow in 2018. In October 2018 I joined the labs of Dr Michael Hubank and Professor Andrea Sottoriva to investigate the use of liquid biopsy to monitor clonal frequency and emergence of resistance mutations in paediatric cancers.
Professor Louis Chesler's group have written 113 publications
Most recent new publication 4/2025
See all their publications