Study and Careers
As the UK’s leading academic research centre, The Institute of Cancer Research offers a fantastic work and study environment, great opportunities for development and the chance to make a real difference for cancer patients. We aim to train, recruit and develop the best – with positions for outstanding scientists and clinicians, and the most talented professional or administrative staff.
Studying at the ICR
Our competitive programmes and specialised courses are designed for the next generation of cancer researchers and clinicians.

PhD projects and opportunities
We offer 20 fully funded PhD studentships each year. Our main round opens in October, but we also advertise projects throughout the year.
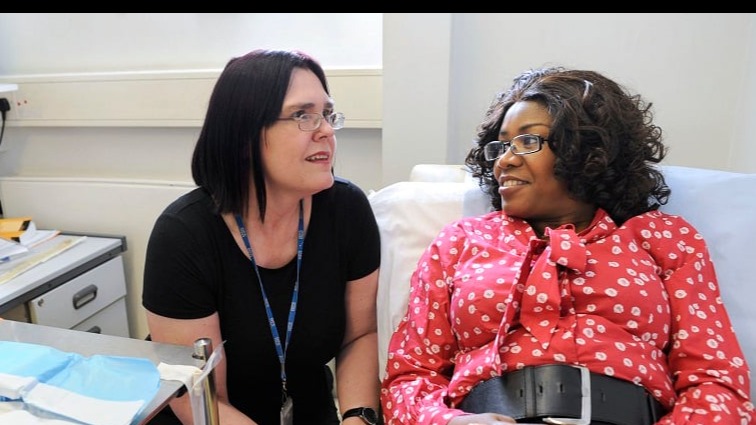
Opportunities for clinicians
At The Institute of Cancer Research, London, we offer clinicians a variety of opportunities – from a taught master's course in Oncology, to fellowships providing protected time for research, and higher research degrees.
MSc in Oncology
The Taught Course in Oncology is a day-release modular programme designed for medically qualified candidates who intend to pursue a professional career in some aspect of clinical or medical oncology, either as a clinical academic or a clinician.
Why study with us?
By providing world-class expertise and support to students at The Institute of Cancer Research, we hope to advance cancer research and clinical practice not only within our organisation, but throughout the field.

Student Profile
Varun Ramaswamy is a third-year PhD student at the ICR. He is working on a collaborative project between the Division of Cancer Therapeutics and the Division of Structural Biology that aims to solve the 3D structure of a protein called HSET using cryo-electron microscopy and complementary biophysical techniques.

Employee Story
Head of Research Support (corporate role) Dr Becky Cook Dr Becky Cook is Head of Research Support at The Institute of Cancer Research. She oversees our large strategic grant applications and is supporting the ICR's next submission to the Research Excellence Framework (REF), which is the definitive government evaluation of the quality and impact of research at UK universities.

Support moving to the UK
Whether you're a student or a full time employee, you'll get a variety of social, welfare and accommodation benefits and advice, to help your move to the UK.
Working life and benefits
As a world-leading cancer research organisation, we are a dynamic and exciting place to work - with various benefits and support if you’re moving to the UK.
Current openings
We offer a fantastic working environment, great opportunities for career development and the chance to make a real difference for cancer patients. We aim to recruit and develop the best – with positions for postdocs, scientists and clinicians, and professional or administrative staff - see the latest below:
Research Group Leader, ICR Clinical Trials and Statistics Unit (ICR-CTSU)
Department/Directorate Information: Division of Clinical Studies - Clinical Trials and Statistics Unit (ICR-CTSU) The ICR-CTSU is a Cancer Research UK-funded, internationally recognised methodologist led clinical trials unit, providing cancer-focused clinical trial research expertise. We lead pioneering, efficient, high-quality, and impactful trials across the phases. Our expertise ranges from experimental medicine early phase studies exploring biological efficacy to trials which may deliver widespread change to routine practice, underpinned by applied methodology to drive forward clinical trial innovation. See our clinical trials Role Summary The Group Leader will lead a component of ICR-CTSU’s portfolio of clinical trials research. The post holder will join an existing faculty and seek to further develop and grow the portfolio in line with ICR-CTSU’s overall strategy; taking responsibility for a number of ongoing trials as well as the development of new trials. There will be the opportunity to develop and grow a team including the potential for a Postdoctoral Training Fellow and/ or a Trial Manager. We seek an experienced statistician / biostatistician with a strong research interest in clinical trials methodology and a passion for direct involvement in the oversight and leadership of academic clinical trials. The successful candidate will work closely with the Director of ICR-CTSU to further enhance the Unit’s internationally recognised strength in clinical trial design, conduct and analysis. The post holder will be expected to make a substantial independent intellectual contribution to clinical trials projects and be proactive in leading and contributing to broad initiatives that enhance the overall effectiveness of ICR-CTSU. The appointee will contribute to the overall scientific life of the ICR including the newly established ICR/Royal Marsden Hospital’s Centre for Trials and Population Data Science, by providing mentorship to more junior colleagues and acting as an academic leader. We seek an individual who will work closely and collaboratively with other faculty/Group Leaders at the ICR and with international/national key opinion leaders to extend the breadth and depth of ICR-CTSU’s biologically rich clinical trials portfolio. In partnership with clinical opinion leaders, this individual will generate research funds to conduct and deliver clinical trials research at the international forefront. Presentation at national and international conferences, production of top-quality research outputs and substantial professional contribution to wider clinical trial network bodies are expected. Enthusiasm for team-based science in a collaborative interdisciplinary environment is essential. The appointment will be based on track record and the ability and willingness to engage in team science. The successful appointee will have access to ICR’s successful PhD training programme and core facilities. Key Requirements Higher degree (MSc or PhD) in medical statistics/biostatistics or an allied field (e.g. public health, epidemiology, data science) with relevant work experience Significant experience as a clinical trials, medical statistician or bio-statistician within the academic or commercial sector; a blend of both would be highly desirable A desire to apply existing and novel statistical methods to the requirements of a diverse range of statistical problems A broad understanding of cancer research Ability to lead a Clinical Trials Unit based research group As part of your online application, you will be required to upload your full CV which will pre-populate your application form, you will also be asked to attach the following documents and failure to do so will mean your application cannot be considered on this occasion: Lists of major publications, achievements, research grants and distinctions. A PDF of a maximum of five key publications, or other research outputs (e.g. patents) that best demonstrate previous productivity or a single document giving hyperlinks to these outputs. You must also complete the personal statement section of the application form in the format of a cover letter including the names and contact details of three academic referees Joining as a Group Leader, you will be given outstanding support to help you to continue to develop in your career. Along with a start-up package of funding, you will also have access to resources to establish your group, including support for recruiting key group members, such as PhD students and postdoctoral researchers. We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Professor Emma Hall ([email protected])
Digital Manager – Website Lead
About the Role We’re looking for a talented Digital Manager to take ownership of the ICR’s main website and lead its evolution. You will manage, optimise and grow our website following its major redevelopment on the latest version of the Sitefinity CMS. This is a pivotal, highly collaborative role at the heart of a busy directorate that tells the ICR’s story and leads our fundraising. Key Responsibilities As our Website Lead, you will: Oversee daily management, development and optimisation of the ICR’s main website — including content, SEO/AEO and technical improvements Work closely with internal partners to develop new pages, sections and features Lead a programme of ongoing content review and user training across the organisation Produce regular website analytics reports and deliver insight-driven recommendations Ensure consistent branding, accessibility and outstanding user experience Manage a Digital Communications Officer (job share, 1.2 FTE) and help recruit and line-manage a new Website Developer Plan and prioritise technical projects with our Digital Services (IT) team About You This is a fantastic opportunity for someone who combines technical understanding with creativity, editorial judgement and a passion for delivering exceptional digital experiences. You’ll bring: Strong experience managing and publishing content within a CMS (Sitefinity experience is a bonus) A solid understanding of HTML and confidence working with developers and IT colleagues Experience overseeing the day-to-day running of a large website Skills in analysing website performance using tools like GA4, Google Tag Manager or Matomo Excellent organisational ability and the skills to manage multiple concurrent projects Strong written and verbal communication skills Experience managing or mentoring others (highly desirable) A proactive, collaborative approach to working across teams Optional but advantageous: experience with Adobe Creative Cloud tools, editorial content review, and training non-technical users What We Offer A supportive and collaborative working environment. Opportunities for professional development and career progression. Competitive salary and pension About the Development and Communications directorate The Development and Communications directorate tells the ICR’s story and focuses on income generation. The ICR is world-renowned for its outstanding cancer research – and it deserves communication to match. We believe that communicating effectively about the ICR's work can help us build on our successes – attracting donors and supporters, the best staff and students, commercial partners and collaborators. We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Eleanor Howard, Head of Strategic Marketing via email on [email protected].
Senior Digital Marketing Manager
We are looking for an experienced and strategic Senior Digital Marketing Manager to lead, as maternity cover, digital marketing activity across the directorate and help drive fundraising growth, brand awareness and supporter engagement. You will shape and deliver our digital marketing strategy, using insight, creativity and analytical rigour to ensure our website, donation platform, email marketing and digital campaigns support the ICR’s fundraising and brand objectives effectively. This is a key leadership role that manages two Digital Marketing Officers and oversees relationships with our external media agency. You will combine deep digital marketing expertise with excellent project management and a passion for impactful storytelling. Key Responsibilities Lead and deliver an annual programme of digital marketing and online supporter engagement to grow income and brand awareness. Act as the digital marketing expert for all fundraising teams, analysing data and proactively sharing insight. Manage paid media campaigns, email programmes, stewardship journeys and digital content planning. Agree and achieve KPIs for audience engagement, online giving and lead generation. Shape supporter journeys to maximise engagement, recruitment and fundraising participation. Manage the relationship with our digital media agency, from planning to evaluation. Optimise the ‘Support Us’ section of the website, leading SEO and AEO activity. Oversee and optimise our donation platform (goDonate), including testing and analytics. Manage a digital marketing budget of more than £300K. Provide digital marketing expertise across the ICR, including email and content support. Ensure strong brand consistency across all digital marketing and fundraising touchpoints. About You You will bring: Strong analytical and problem-solving skills, with experience using SEO, Google Ads, Google Analytics and evaluation tools. Expertise across the full digital marketing mix. Excellent leadership skills, including line management. Ability to build strong relationships internally and externally. Understanding of digital tech including HTML basics, tracking pixels and marketing tags. Excellent written communication and strong design sense. Highly organised, with the ability to manage multiple projects simultaneously. Genuine enthusiasm for the ICR’s mission. Proven track record managing digital campaigns from conception to delivery. Experience managing agencies. Experience using data and insight to optimise campaigns and maximise income. Experience in content generation, website management, paid media and social platforms. Experience with online giving platforms. What We Offer A supportive and collaborative working environment. Opportunities for professional development and career progression. Competitive salary and pension About the Development and Communications directorate The Development and Communications directorate tells the ICR’s story and focuses on income generation. The ICR is world-renowned for its outstanding cancer research – and it deserves communication to match. We believe that communicating effectively about the ICR's work can help us build on our successes – attracting donors and supporters, the best staff and students, commercial partners and collaborators. We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Talent Team on [email protected]
Higher Scientific Officer
Under the guidance of Alexis De Haven Brandon, we are seeking to recruit a Higher Scientific Officer. We seek an experienced and dedicated in vivo scientist. The central aim of this position is to undertake in vivo technical work, playing a key role in supporting a variety of in vivo experiments and laboratory approaches. You will be a highly skilled in vivo scientist with sound knowledge and experience in in vivo approaches in a research setting. You will possess a Home Office Personal Licence. Your responsibilities will include planning and performing animal procedures, maintaining detailed records, and performing some routine laboratory tasks. This position offers an excellent opportunity for a highly skilled in vivo scientist with a passion for in vivo science, and animal welfare. There will occasionally be the need for out of hours work to ensure Home Office compliance, and the needs of the projects are met. The successful candidate will be a motivated individual with a strong background in in vivo techniques. We highly value diversity and encourage candidates from all backgrounds to apply. About you The successful candidate must have: Personal Home Office Licence PIL A,B,C BSc in life sciences or equivalent Proven experience in a technical in vivo research setting - to include techniques such as dosing, surgical and sampling Ability to plan and execute complex in vivo studies, to the highest ethical standards Ability to work independently and collaboratively Department/Directorate Information The In Vivo Pharmacology team (IVP) provides in vivo support for all projects within the Centre for Cancer Drug Discovery (CCDD). The team carries out critical proof-of principle intervention studies and provides the essential pharmacological assays required to guide target validation in addition to the optimisation and selection of new drug candidates. Importantly, these experiments include the demonstration of drug effects in vivo, biomarker discovery and therapy studies, all of which underpin the drug discovery programs across the portfolio. The IVP works across a wide range of tumour models, including human tumour xenografts models (both cell line- and patient-derived xenografts), genetically engineered mouse models, organoid-based models and preclinical metastatic models. The team has specialised skills to develop and implement novel models, and supporting the design and implementation of studies, whilst complying with increasingly complex Home Office regulations. What we offer A dynamic and supportive research environment Access to state-of-the-art facilities and professional development opportunities Collaboration with leading researchers in the field Competitive salary and pension We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Alexis De Haven Brandon: [email protected].
