
CLOSED: Structural dissection of β-catenin ubiquitylation by cryo-electronmicroscopy
Application closing date: 16/10/25
Project background
The Wnt/β-catenin signalling pathway is a fundamental regulator of embryonic development, tissue homeostasis, and regeneration1. Central to this pathway is the β-catenin destruction complex (DC), a multi-protein assembly that orchestrates the phosphorylation and subsequent ubiquitylation of β-catenin, earmarking it for proteasomal degradation (Figure 1A)2,3. Dysregulation of the DC is a hallmark of several cancers, most notably colorectal cancer (CRC), where mutations in the tumour suppressor adenomatous polyposis coli (APC) and the protooncogene β-catenin are prevalent. Disruptions in axis inhibition protein 1 (AXIN1), the DC’s central scaffolding subunit, are observed in hepatocellular and endometrial carcinomas4,5.
Despite its clinical relevance, the Wnt/β-catenin pathway remains untargeted by approved therapeutics. This is largely due to (mostly stem-cell directed) toxicity associated with pathway inhibition, the pathway’s mechanistic complexity, and a lack of structural/mechanistic insights into its regulation to reveal novel targeting opportunities6. A deeper understanding of the molecular architecture and regulation of the complexes controlling the pathway, including the DC, is essential to both understand fundamental molecular mechanisms and identify novel therapeutic nodes.
The DC’s scaffolding proteins APC and AXIN1 co-recruit β-catenin along with casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β). These kinases sequentially phosphorylate β- catenin at a phosphodegron, thereby enabling β-catenin’s recognition by the SCFβ-TrCP E3 ubiquitin ligase complex7, which gives rise to polyubiquitylation and subsequent proteasomal degradation of β-catenin2,3.
Recent work in the laboratory has led to the successful reconstitution of the DC from purified proteins and recapitulated that AXIN1 self-assembly and APC are critical, both for efficient β- catenin phosphorylation and ubiquitylation8. Notably, we discovered a direct interaction between APC and SCFβ-TrCP, suggesting a previously unappreciated regulatory role for APC in modulating β-catenin degradation. However, the structural basis and functional consequences of this interaction remain unknown. Likewise, other potential interaction points between the DC and SCFβ-TrCP, underpinning the formation of a potential super-complex, remain unexplored9–13.
This proposal seeks to dissect the structural details of mechanisms governing β-catenin ubiquitylation within the DC using cryo-electron microscopy (cryo-EM), complemented by biochemical and proteomics approaches. The central hypotheses are (1) that the architecture of the DC:SCFβ-TrCP super-complex directs ubiquitylation site choice, with consequences for how β- catenin is corralled through the complex, and (2) that APC, and potentially AXIN1, not only scaffold the DC but also facilitate the communication between the DC and SCFβ-TrCP for efficient β-catenin processing. An in-depth structural characterisation of these complexes will illuminate whether and how APC truncations, which occur in CRC, dysregulate the normal dialogue between the complexes.
The candidate, funded by Cancer Research UK, will join a multidisciplinary, supportive team dedicated to investigating the mechanisms of normal and oncogenic Wnt/β-catenin signalling. The project will provide extensive training in biochemistry, biophysics and single-particle cryo- EM, with opportunities to gain experience in proteomics (including structural proteomics) through collaborations.
Project aims
- Determine cryo-EM structures of SCFβ-TrCP in complex with components of the DC, paired with structural proteomics
- Reveal how DC components, particularly APC, interact with the β-catenin ubiquitylation machinery
- Visualise substrate-engaged states of SCFβ-TrCP
- Capture intermediates to elucidate how β-catenin is positioned for ubiquitylation
- Integrate cryo-EM with biochemical assays to map functional interfaces
- Identify key contact points and elucidate how they regulate β-catenin modification and turnover
- Assess consequences of APC truncation on complex assembly
- Understand whether and how cancer-associated mutations alter β-catenin ubiquitylation
Further details & requirements
Our recent work has shown that APC directly interacts with SCFβ-TrCP and promotes β-catenin ubiquitylation, modulating processivity8, but the structural basis of this interaction and its precise mechanistic impact remain unknown. We also lack structural insights into how other components of the DC, most notably AXIN1, may facilitate the dialogue with the ubiquitylation machinery9–13. Over the last decade, cryo-EM has transformed our ability to resolve the architecture of large, dynamic protein complexes. This project aims to use cryo-EM to dissect the structural mechanisms of β-catenin ubiquitylation in the context of a DC:SCFβ-TrCP super-complex.
Project Objectives
1. Investigating the APC:SCFβ-TrCP complex
To investigate how APC promotes β-catenin ubiquitylation, the candidate will study the structure of APC:SCFβ-TrCP complex variants using single-particle cryo-EM. This will entail target expression in the baculovirus-insect cell system and the subsequent purification and assembly of complexes (Figure 1B). Prior to structural characterisation, the relevant complexes will be analysed by biophysical techniques, including size exclusion chromatography coupled to multiangle light scattering (SEC-MALS) and mass photometry.
The candidate will next turn to characterising the complexes by EM. Initial explorations by negative-stain EM will be followed by cryo-EM, which offers opportunities to build experience in dealing with common challenges such as maintaining sample integrity at the air-water interface and preferred orientation14. Following hands-on or remote data collection, using the in-house Glacios TEM, or Titan Krios TEMs available through the London Consortium for Cryo-EM (LonCEM) or the Electron Bio-Imaging Centre (eBIC) at the Diamond Light Source, the candidate will gain extensive experience in cryo-EM data processing, structural model building and interpretation. This work will be supported by Team Members and Facility Managers. Opportunities for parallel, complementary structural proteomics studies (by crosslinking mass spectrometry and hydrogen-deuterium exchange mass spectrometry15), in collaboration with experts in these areas, are available.
Structural insights into APC:SCFβ-TrCP interactions will guide site-directed mutagenesis to modulate identified binding interfaces. The candidate will next probe the functional relevance of these interfaces through in vitro β-catenin phosphorylation and ubiquitylation assays with the reconstituted DC8 (Figure 1C).
2. Expanding the complex to understand the mechanism of β-catenin ubiquitylation
Accompanying the characterisation of the APC:SCFβ-TrCP complex, the candidate will build up the assemblies further, to explore the mechanistic details of β-catenin ubiquitylation. Additional components will include NEDD8 on CUL116, E2 ubiquitin conjugating enzymes (e.g., UBCH5, CDC34), full-length phosphorylated β-catenin, and ubiquitin. Established approaches to trap transient states for subsequent structural studies by cryo-EM and structural proteomics will be used17. Structural studies will be followed by site-directed mutagenesis and functional studies in vitro, as outlined above. The candidate will collaborate with Professor Jyoti Choudhary (Functional Proteomics Group and ICR Proteomics Core Facility) to map ubiquitylation sites within β-catenin by mass spectrometry. Studying structure-based mutant variants of the system will enable the candidate to relate ubiquitylation complex architecture to ubiquitylation site specificity.
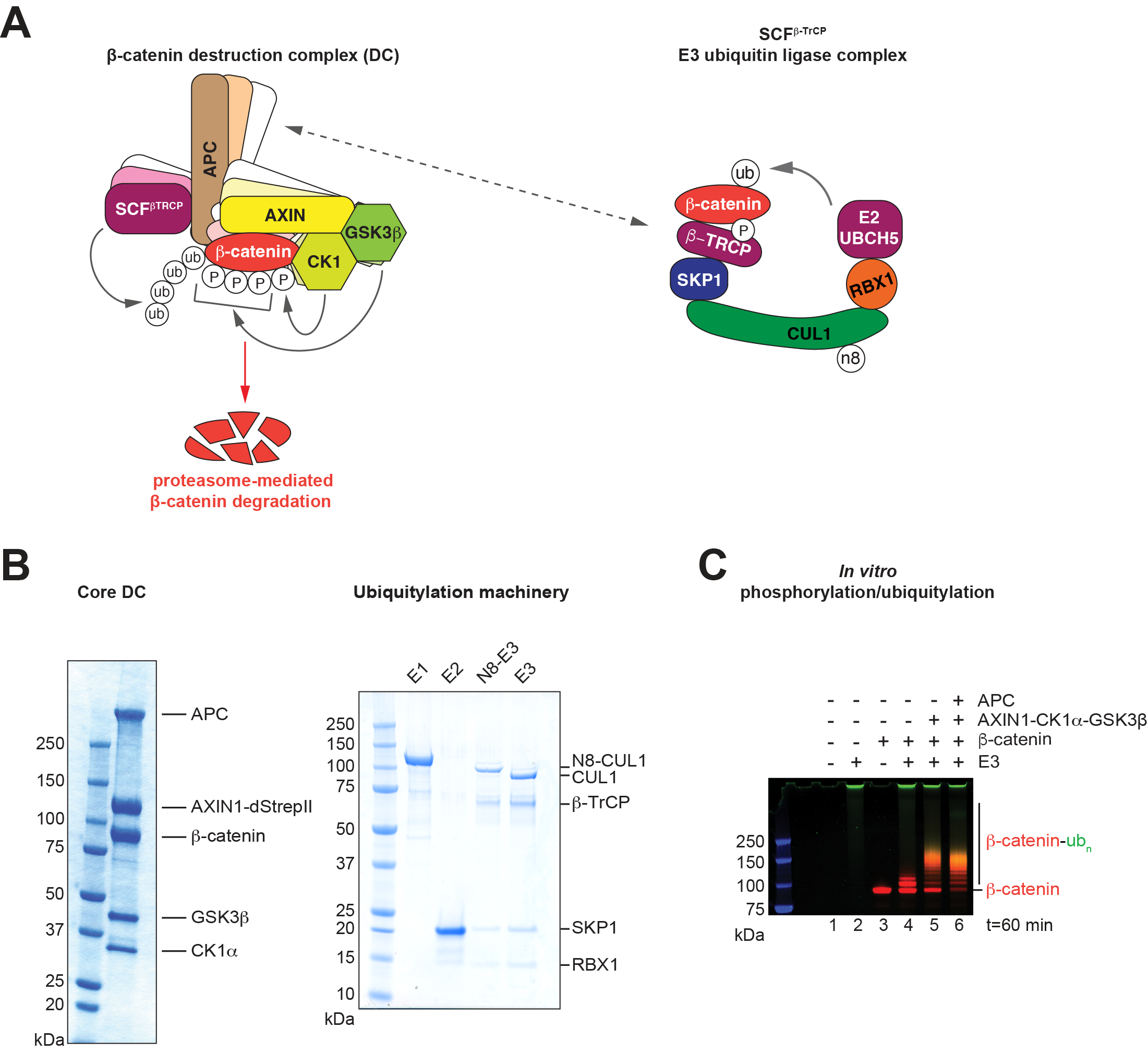
Figure 1: Mechanism and function of the APC:SCFβ-TrCP interaction. (A) Schematic representation of the DC and SCFβ-TrCP. (B) SDS-PAGE/Coomassie analysis of components of the DC and ubiquitylation machinery (E1 ubiquitin-activating enzyme UBA1, E2 ubiquitin-conjugating enzyme UBCH5C, E3 composed of CUL1, β-TrCP, SKP1 and CUL1 and its NEDDylated (N8) variant). (C) Fluorimetric in vitro ubiquitylation assay for β-catenin. Ubiquitin is labelled with FITC, β-catenin with Alexa Fluor 680. Images were adapted from reference 8.
Perspective and Impact
This project will provide the first high-resolution structural insights into β-catenin ubiquitylation within the wider DC:SCFβ-TrCP system. By elucidating how APC mediates the communication between the DC and the E3 ligase and how full-length β-catenin is engaged, the work will advance our understanding of Wnt/β-catenin signalling and its dysregulation in cancer. We anticipate that insights will inform novel strategies to modulate β-catenin stability, eventually opening up new avenues for targeted cancer therapy.
Note: the ICR’s standard minimum entry requirement is a relevant undergraduate Honours degree (First or 2:1).
Pre-requisite qualifications of applicants:
- BSc or MSc in Biochemistry, Biophysics or Structural Biology with demonstrable laboratory experience
Intended learning outcomes:
- Mastery of single-particle cryo-EM (sample preparation, data collection, processing, model building and interpretation) • Expertise in protein biochemistry/biophysics (expression, purification, reconstitution, binding assays, in vitro phosphorylation and ubiquitylation, SEC-MALS, mass photometry)
- Skills in proteomics sample preparation and data analysis
- Expertise in Wnt/β-catenin signalling and knowledge of cancer biology
- Cross-disciplinary data integration from structural mechanisms to cellular phenotypes
1. Rim, E. Y., Clevers, H. & Nusse, R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu Rev Biochem 91, 571–598 (2022).
2. Stamos, J. L. & Weis, W. I. The β-catenin destruction complex. Cold Spring Harbor Perspectives in Biology 5, a007898–a007898 (2013).
3. Kappel, E. C. van & Maurice, M. M. Molecular regulation and pharmacological targeting of the β-catenin destruction complex. Brit J Pharmacol 174, 4575–4588 (2017).
4. Sanchez-Vega, F. et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 173, 321-337.e10 (2018).
5. Bugter, J. M., Fenderico, N. & Maurice, M. M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer 21, 5–21 (2021).
6. Zhang, Y. & Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 13, 165 (2020).
7. Wu, G. et al. Structure of a β-TrCP1-Skp1-β-Catenin Complex. Molecular Cell 11, 1445–1456 (2003).
8. Ranes, M., Zaleska, M., Sakalas, S., Knight, R. & Guettler, S. Reconstitution of the destruction complex defines roles of AXIN polymers and APC in β-catenin capture, phosphorylation, and ubiquitylation. Molecular Cell 81, 3246-3261.e11 (2021).
9. Kitagawa, M. et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β- catenin. EMBO J. 18, 2401–2410 (1999).
10. Hart, M. et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9, 207–211 (1999).
11. Schaefer, K. N. et al. Wnt regulation: exploring Axin-Disheveled interactions and defining mechanisms by which the SCF E3 ubiquitin ligase is recruited to the destruction complex. Mol. Biol. Cell 31, 992–1014 (2020).
12. Liu, C. et al. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc. Natl. Acad. Sci. 96, 6273–6278 (1999).
13. Li, V. S. W. et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell 149, 1245–1256 (2012).
14. Wu, M. & Lander, G. C. Present and Emerging Methodologies in Cryo-EM Single-Particle Analysis. Biophys. J. 119, 1281–1289 (2020).
15. Happonen, L. J. & Varjosalo, M. State-of-the-Art and Future Directions in Structural Proteomics. Mol. Cell. Proteom. 24, 101065 (2025).
16. Baek, K. et al. NEDD8 nucleates a multivalent cullin-RING-UBE2D ubiquitin ligation assembly. Nature 578, 461–466 (2020).
17. Henneberg, L. T. & Schulman, B. A. Decoding the messaging of the ubiquitin system using chemical and protein probes. Cell Chem. Biol. 28, 889–902 (2021).