
CLOSED: Regulation of the β-catenin destruction complex by deubiquitylation
Application closing date: 16/11/25
Project background
The Wnt/β-catenin signalling pathway is a fundamental regulator of embryonic development, tissue homeostasis, and regeneration1. Central to this pathway is the β-catenin destruction complex (DC), a multi-protein assembly that orchestrates the phosphorylation and subsequent ubiquitylation of β-catenin, marking it for proteasomal degradation (Figure 1A)2,3. Dysregulation of the DC is a hallmark of several cancers, most notably colorectal cancer (CRC), where mutations in the tumour suppressor adenomatous polyposis coli (APC) and the proto-oncogene β-catenin are prevalent. Mutations in axis inhibition protein 1 (AXIN1), the DC’s central scaffold, are observed in hepatocellular and endometrial carcinomas4,5.
Despite its clinical relevance, the Wnt/β-catenin pathway remains untargeted by approved therapeutics. This is largely due to toxicity associated with pathway inhibition (particularly towards tissue stem cells), but also the pathway’s mechanistic complexity and a lack of structural/mechanistic insights into its regulation6. A deeper understanding of the molecular architecture and regulation of the complexes governing the Wnt/β-catenin signalling pathway, including the DC, is essential to both appreciate fundamental signalling mechanisms and identify novel therapeutic intervention points.
The DC’s scaffolding proteins APC and AXIN1 co-recruit β-catenin along with casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β). These kinases sequentially phosphorylate β- catenin, priming it for recognition by the SCFβ-TrCP E3 ubiquitin ligase complex7, which gives rise to polyubiquitylation and subsequent proteasomal degradation of β-catenin2,3. APC and AXIN1 are also subject to CK1- and GSK3β-dependent phosphorylation8–10.
Recent work in our laboratory has led to the successful reconstitution of the DC from purified proteins, which opened new opportunities for investigating the mechanism and regulation of the DC11.
Regulatory post-translational modifications tend to be short-lived and are often rapidly reversed by erasers that serve to reset signalling systems. The de-ubiquitylating enzyme (DUB) USP7 was shown to counter β-catenin ubiquitylation, thereby stabilising β-catenin and acting as a positive regulator of Wnt/β-catenin signalling12. In Apc-mutant mice, Usp7 deletion or pharmacological inhibition suppresses intestinal carcinogenesis13. USP7 was proposed directly bind to the β- catenin N-terminus at a site close to the phosphodegron recognised by β-TrCP12. APC truncation favours USP7 binding, at the expense of β-TrCP binding12, which makes USP7 a promising tumour-specific target13,14. However, the mechanism underlying the antagonism between the ubiquitylation writer and eraser and the coordination of their activities remain unknown. Adding further complexity to the system, a study by Cong and colleagues suggests that rather than β- catenin, the primary target of USP7 in the DC is AXIN1, at least in the context of wild-type APC15. Here, USP7 constitutes a negative rather than positive regulator of Wnt/β-catenin signalling as its activity would lead to AXIN1 stabilisation, resulting in increased DC formation and function. The USP7 binding sites in AXIN1 have not yet been charted conclusively, and whether this target specificity is maintained in an APC truncation context remains unknown. Furthermore, a recent study identified yet another DUB, namely USP10, as a positive regulator of β-catenin levels and activity, specifically in the context of APC truncation16. USP10 employs its unstructured N-terminus to bind β-catenin, presumably at sites overlapping with those for APC, which offers a potential explanation for its tumour-specific effects16. Taken together, this indicates a clear role of DUBs in controlling the function of the DC.
This proposal seeks to investigate the precise mechanisms by which USP7 and USP10 engage the DC and subsequently de-ubiquitylate their targets in the complex, both in the context of wildtype and truncated APC. The central hypotheses are (1) that recruitment/substrate specificities of these DUBs switch depending on APC status and (2) that competing interactions in the DC account for context-specific functions of these DUBs.
The candidate, funded by Cancer Research UK, will join a multidisciplinary, supportive team dedicated to investigating the mechanisms of normal and oncogenic Wnt/β-catenin signalling. The project will provide extensive training in biochemistry, biophysics and structural studies by Xray crystallography, alongside experience in cell biology and proteomics gained through collaboration.
Project aims
- Determine the target specificity of USP7 and USP10 within the DC in wild-type and mutant APC context at amino-acid resolution
- Understand the context-specific targeting of the DC by DUBs
- Characterise the binding modes of the DUBs using biophysical assays and X-ray crystallography
- Understand the mechanistic basis for context-specific functions of the DUBs
- Develop isogenic cell systems to probe the functions of USP7 and USP10 in normal and oncogenic Wnt/β-catenin signalling
- Investigate context-specific mechanisms and identify
Further details & requirements
Project Objectives
1. Investigating target specificity of USP7 and USP10
Previous studies indicate that APC truncation extensively changes in the functions of USP7 and USP10 towards the DC12,15,16. To explore such switches in a reductionist system, the candidate will express and purify full-length variants of the DUBs (Figure 1B), using the baculovirus-insect cell system, for subsequent activity assays. These will be performed with the reconstituted DC, as previously reported11 (Figure 1C). DC variants will either lack APC, include wild-type APC or CRC-associated truncation variants. Following in vitro phosphorylation and ubiquitylation, USP7 and USP10 will be supplied and ubiquitylation status monitored by fluorimetry and Western blotting, which will provide first insights into target specificity. To obtain a comprehensive picture of target specificity at amino-acid resolution, the candidate will collaborate with Professor Jyoti Choudhary’s team (Functional Proteomics Group and Proteomics Core Facility) to map ubiquitylation sites by quantitative mass spectrometry. This will generate a resource for understanding context-specific function of these DUBs in the DC. DUB target sites will likely not be limited to β-catenin but also occur within other DC components11, which will point towards novel hypotheses regarding the regulation of the DC.
2. Structural and biophysical characterisation of USP7/10 interactions with DC components
There is currently no experimental structural information available describing the interaction of USP7 or USP10 with components of the DC. AlphaFold predicts that the unstructured Nterminus of USP10 interacts with β-catenin at sites engaged by other DC components (AXIN1, APC)16. The candidate will use bioinformatics and structural modelling to identify candidate USP7/10-binding motifs within the DC and subsequently validate these using fluorescence polarisation (FP) assays. This will include binding competition studies. Selected interactions will next be characterised by X-ray crystallography, supported by the Biophysics and Crystallisation Facility (Dr Stephen Hearnshaw). Diffraction data will be collected at the Diamond Light Source synchrotron. Structural data will enable the engineering of precise mutations to modulate the relevant interactions for subsequent functional dissection using purified proteins and cell-based systems.
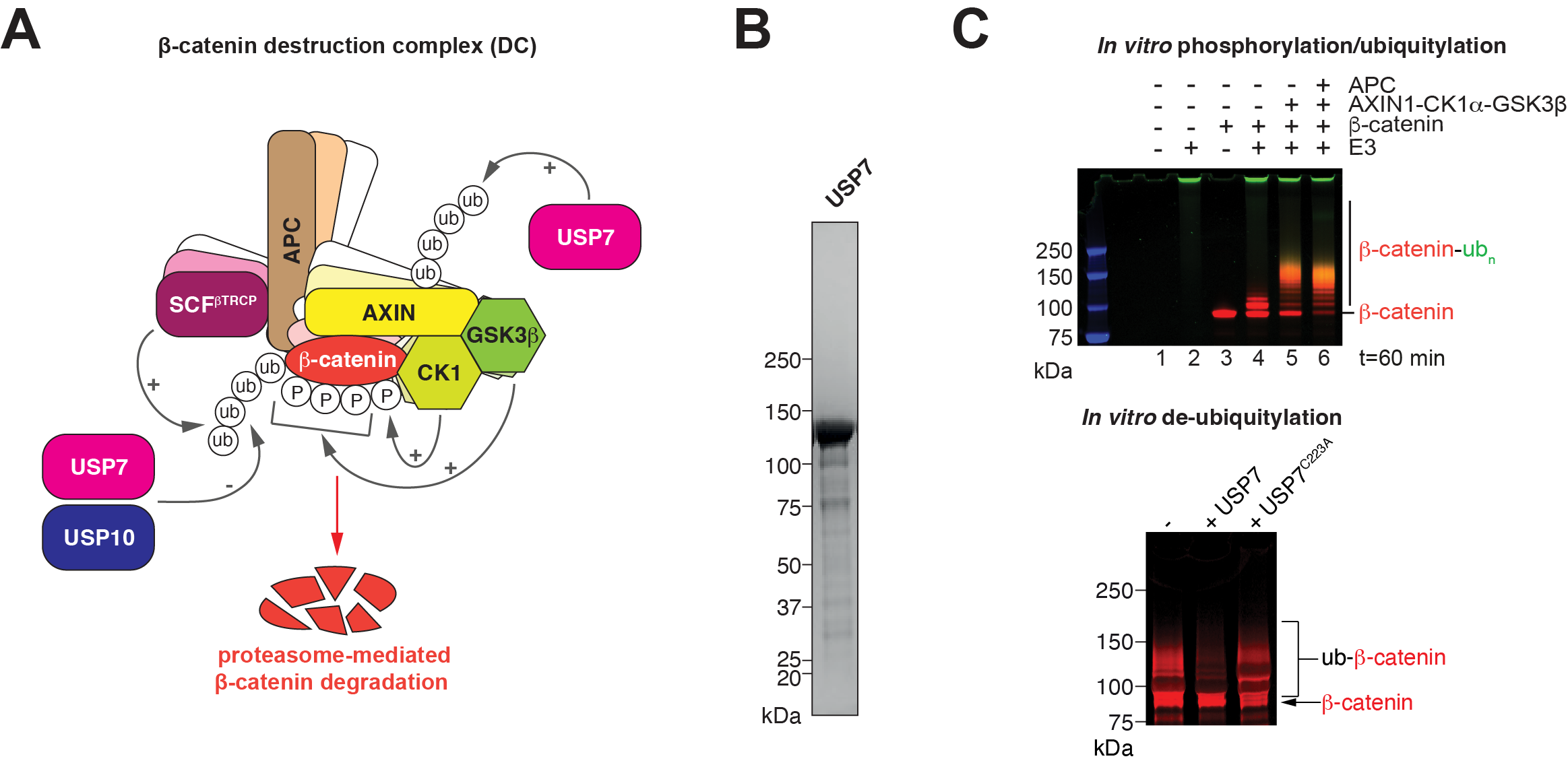
Figure 1: Ubiquitylation and de-ubiquitylation in the DC.(A) Schematic representation of the DC with ubiquitylation and de-ubiquitylation activities by the indicated enzymes. (B) Purified full-length USP7 analysed by SDS-PAGE and Coomassie staining. (C) Top, fluorimetric in vitro ubiquitylation assay for β-catenin. Ubiquitin is labelled with FITC (shown in green), β-catenin with Alexa Fluor 680 (shown in red). Bottom, de-ubiquitylation of β-catenin by USP7. C223A is an inactive mutant variant of USP7. Images were in part adapted from reference 11.
3. Development of isogenic cell models to probe the context-dependent functions of DUBs in the DC
The candidate will next develop cellular models for probing the functions and mechanisms of the DUBs, supported by a Cell Biologist within the team. We will turn to HAP1 cells, which are exquisitely suited for genetic perturbations given their haploid genotype17 and have previously been used to investigate the mechanisms of Wnt/β-catenin signalling18, including in our team (unpublished). HAP1 cells with precisely engineered APC truncation status will be generated. The candidate will use CRISPR knockout and small-molecule USP7/10 inhibitors to abrogate/modulate DUB function, followed by analyses of gene expression, monitoring either a β-catenin-responsive transcriptional reporter (Figure 2) or endogenous β-catenin target genes in various APC backgrounds. HAP1 cells will facilitate the selective abrogation of USP7/10 binding sites by point mutation, introduced either via homologous recombination19 or Prime Editing20. Findings will be followed up in selected CRC cell models with either wild-type APC or a range of APC truncations.
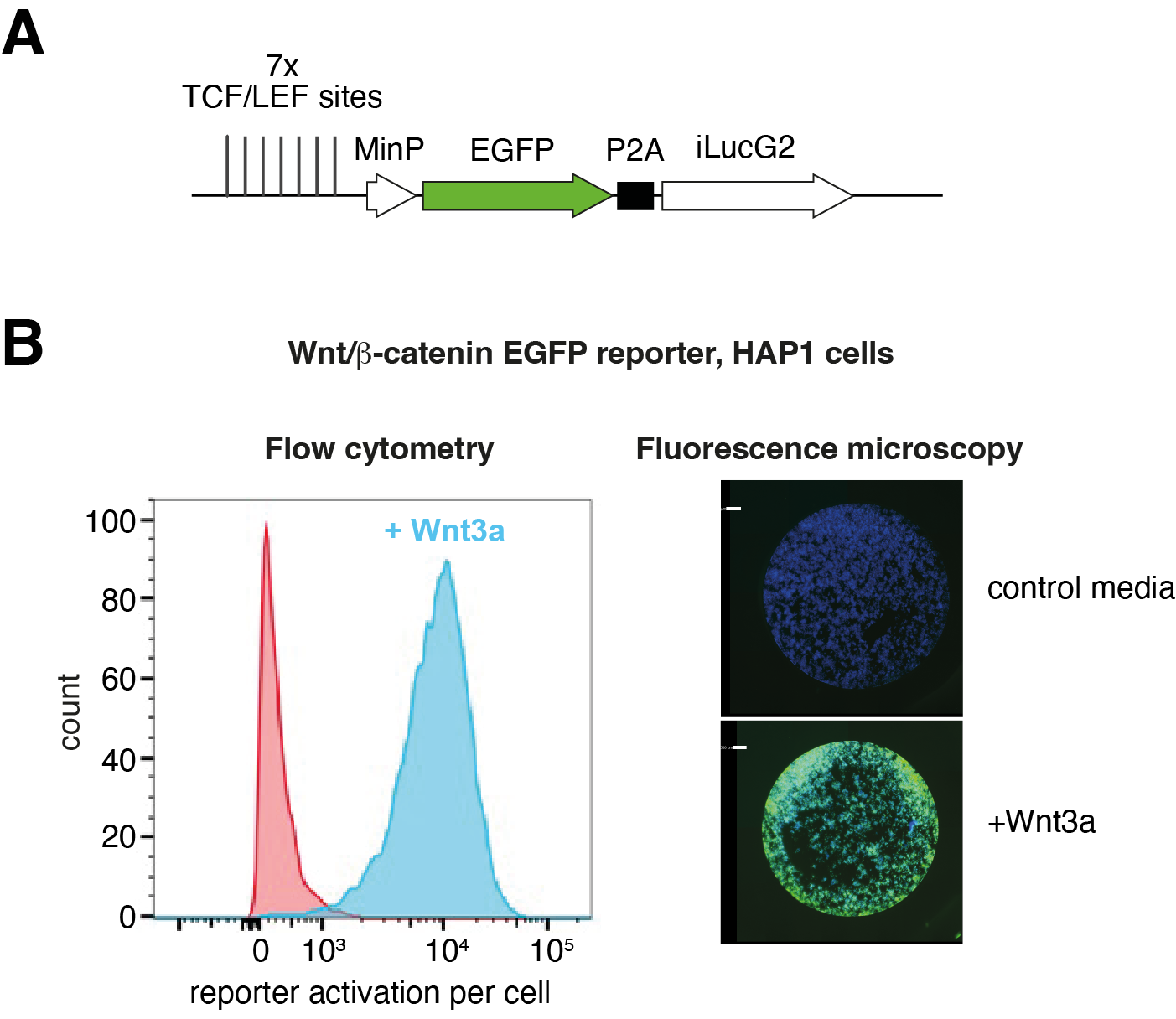
Figure 2: Investigating Wnt/β-catenin signalling in HAP1 cells. (A) Schematic representation of a β-catenin-responsive EGFP reporter. (B) Response of the stably integrated reporter to Wnt3a stimulation of HAP1 cells. Left, flow cytometry; right, fluorescence microscopy (DAPI stains cell nuclei; EGFP shown in green).
Perspective and Impact
Safely targeting oncogenic Wnt/β-catenin signalling remains a formidable challenge as it is important to spare normal Wnt/β-catenin signalling in non-cancer cells (particularly stem cells). Recent work points to USP7 and USP10 as potential tumour-specific targets, but further mechanistic insights are required to explore them as potential therapeutic targets. The proposed project will provide such insights that will guide future efforts to target these DUBs in β-catenindependent cancers.
Note: the ICR’s standard minimum entry requirement is a relevant undergraduate Honours degree (First or 2:1).
Pre-requisite qualifications of applicants:
BSc or MSc in Biochemistry, Biophysics, Structural Biology or Biomedical Sciences with demonstrable laboratory experience
Intended learning outcomes:
- Expertise in protein biochemistry and biophysics (expression, purification, reconstitution, characterisation, enzyme assays)
- Expertise in Structural Biology (X-ray crystallography)
- Competence in proteomics workflows (quantitative mass spectrometry for mapping post-translational modifications)
- Competence in using cell models for functional studies (CRISPR-based genome editing, transcription reporter assays, gene expression analyses)
- Expertise in Wnt/β-catenin signalling and knowledge of cancer biology
- Cross-disciplinary data integration from structural mechanisms to cellular phenotypes
1. Rim, E. Y., Clevers, H. & Nusse, R. The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annu Rev Biochem 91, 571–598 (2022).
2. Stamos, J. L. & Weis, W. I. The β-catenin destruction complex. Cold Spring Harbor Perspectives in Biology 5, a007898–a007898 (2013).
3. Kappel, E. C. van & Maurice, M. M. Molecular regulation and pharmacological targeting of the β-catenin destruction complex. Brit J Pharmacol 174, 4575–4588 (2017).
4. Sanchez-Vega, F. et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 173, 321-337.e10 (2018).
5. Bugter, J. M., Fenderico, N. & Maurice, M. M. Mutations and mechanisms of WNT pathway tumour suppressors in cancer. Nat Rev Cancer 21, 5–21 (2021).
6. Zhang, Y. & Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol 13, 165 (2020).
7. Wu, G. et al. Structure of a β-TrCP1-Skp1-β-Catenin Complex. Molecular Cell 11, 1445–1456 (2003).
8. Spink, K. E., Fridman, S. G. & Weis, W. I. Molecular mechanisms of beta-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-beta-catenin complex. EMBO J 20, 6203–6212 (2001).
9. Liu, J., Xing, Y., Hinds, T. R., Zheng, J. & Xu, W. The third 20 amino acid repeat is the tightest binding site of APC for beta-catenin. J. Mol. Biol. 360, 133–144 (2006).
10. Kim, S.-E. et al. Wnt stabilization of β-catenin reveals principles for morphogen receptorscaffold assemblies. Science 340, 867–870 (2013).
11. Ranes, M., Zaleska, M., Sakalas, S., Knight, R. & Guettler, S. Reconstitution of the destruction complex defines roles of AXIN polymers and APC in β-catenin capture, phosphorylation, and ubiquitylation. Molecular Cell 81, 3246-3261.e11 (2021).
12. Novellasdemunt, L. et al. USP7 Is a Tumor-Specific WNT Activator for APC-Mutated Colorectal Cancer by Mediating β-Catenin Deubiquitination. Cell Reports 21, 612–627 (2017).
13. Novellasdemunt, L. et al. USP7 inactivation suppresses APC-mutant intestinal hyperproliferation and tumor development. Stem Cell Rep 18, 570–584 (2023).
14. Schauer, N. J. et al. Selective USP7 inhibition elicits cancer cell killing through a p53- dependent mechanism. Sci. Rep. 10, 5324 (2020).
15. Ji, L. et al. USP7 inhibits Wnt/β-catenin signaling through promoting stabilization of Axin. Nat Commun 10, 4184 (2019).
16. Reissland, M. et al. USP10 drives cancer stemness and enables super-competitor signalling in colorectal cancer. Oncogene 43, 3645–3659 (2024).
17. Llargués-Sistac, G., Bonjoch, L. & Castellvi-Bel, S. HAP1, a new revolutionary cell model for gene editing using CRISPR-Cas9. Front. Cell Dev. Biol. 11, 1111488 (2023).
18. Lebensohn, A. M. et al. Comparative genetic screens in human cells reveal new regulatory mechanisms in WNT signaling. Elife 5, (2016).
19. Agudelo, D. et al. Marker-free coselection for CRISPR-driven genome editing in human cells. Nat. Methods 14, 615–620 (2017).
20. Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).