.tmb-hbdesktop.jpg?Culture=en&sfvrsn=78365c0a_1)
CLOSED: Enabling Oral Delivery of VHL-based PROTACs Through Formulation and Prodrug Design
Application closing date: 16/11/25
Project background
Proteolysis-targeting chimeras (PROTACs), particularly those incorporating the von Hippel–Lindau (VHL) E3 ligase ligand, present significant physicochemical challenges due to their large molecular weight, poor aqueous solubility, and low membrane permeability. As a result, oral bioavailability remains a major hurdle, and all clinical-stage VHL PROTACs to date are administered intravenously.
Formulation offers a potential solution – however, formulation science for PROTACs remains underexplored, with limited systematic investigation into the effectiveness of diverse and evolving array of formulation technologies.
In drug discovery, formulation considerations are often deferred until late-stage candidate selection, when compound optimisation is complete and physicochemical liabilities are already embedded. Furthermore, lack of consideration of formulation strategies in early discovery frequently results in suboptimal solubility during preclinical studies, leading to dosing challenges and compromised pharmacokinetic profiles.
By developing a rational understanding of how different formulation approaches perform across compounds with varying physicochemical properties, we can design PROTAC molecules with formulation in mind—combining permeability, solubility, and stability to enable improved systemic exposure following oral administration, supporting the progression of next-generation medicines through preclinical research and towards patient benefit.
Project aims
- Develop an understanding of which formulation strategies are effective at improving solubility for VHL PROTACs with diverse physicochemical properties
- Design and evaluate VHL-based prodrugs to improve cell permeability by modulating hydrogen-bonding and lipophilicity.
- Integrate solubility-enhancing formulations with permeability-enhancing prodrug approaches to rationally select and optimisation PROTAC–prodrug–formulation combinations with improved in vitro and in vivo performance.
- Establish a formulation toolkit for early-stage PROTAC optimisation, enabling rapid screening and progression to preclinical proof-of-concept
- Explore and compare the effectiveness of advanced delivery platforms (e.g. SEDDS, lipid carriers, polymeric nanoparticles) to overcome oral bioavailability barriers for VHL PROTAC
Further details & requirements
Overview:
VHL-based PROTACs represent a promising therapeutic modality, but their physicochemical properties limit potential for oral delivery. We propose a rational approach to integrate formulation science into early-stage PROTAC design, enabling improved systemic exposure.
1. Compound Selection and Initial Formulation Screening:
Firstly, we will select 10-20 PROTAC molecules from in-house programs and literature sources spanning a range of physicochemical properties, including molecular weight and polarity, with low metabolic clearance. Each compound will be screened against up to 10 formulation types representing diverse chemistries. Solubility and chemical stability will be measured using in house methods in aqueous and biorelevant media (FASSIF/SGF). This dataset will build an understanding of which approaches are effective for different compound classes, and define a minimum screenable formulation set, enabling rapid identification of appropriate formulations
2. Design and Synthesis of VHL Prodrugs
To address permeability limitations, we will synthesise prodrugs which enhance permeability through modulating lipophilicity and blocking hydrogen-bond donor groups on the VHL warhead. We will mitigate solubility reduction by considering molecular shape – e.g. induction of hydrophobic collapse to reduce exposed lipophilic surface. Assessing permeability improvements can be challenging for PROTACs, with PAMPA assays proving unreliable. We will therefore explore alternatives such as biomimetic chromatography, and modified Caco-2 assay conditions. In house assays will be used to compare VHL-target engagement kinetics in intact or lysed cells providing a measure of permeability. Solubility and stability will be assessed using LC-MS, and plasma stability assays applied to evaluate prodrug release rate.
3. Screen excipients to enhance the solubility of PROTAC molecules developed in previous stage
Following the creation of a range of prodrugs, we will screen excipients to identify combinations that enhance solubility. This builds on insights from initial compound screening, allowing prioritisation of excipients most likely to be compatible with the physicochemical profiles of selected PROTACs. Stability and solubility of excipient-PROTAC combinations will be assessed, both neat and upon aqueous dilution. Promising combinations will be profiled through low-dose pharmacokinetic experiments to assess progress towards oral bioavailability.
The goal is to identify formulation approaches with strong potential for further development, serving as a foundation for more advanced formulation design in subsequent stages of the project.
4. Design novel formulations using combination of excipients and advanced delivery platforms
Building on the solubility and permeability data generated in the previous stage, we will design formulations to improve the solubility of selected PROTACs. Initial efforts will focus on designed combinations of excipients based on simple systems such as aqueous solutions, suspensions, and emulsions, which can be prepared and assessed quickly at small scale. At this stage, the effectiveness of permeation enhancing excipients can be assessed.
In parallel, we will explore more advanced delivery platforms selected based on compound-specific challenges. These could include self-emulsifying drug delivery systems (SEDDS), lipid-based carriers, and polymeric nanocarriers. These platforms offer advantages in overcoming the solubility, permeability and stability challenges associated with PROTACs. For example, polymeric nanocarriers can improve solubility, and through encapsulation, protect compound from degradation in the GI tract. Formulations will be prepared using techniques such as microfluidics or high-pressure homogenisation and characterised using dynamic light scattering (DLS), zeta potential analysis, HPLC, and encapsulation efficiency measurements. Visual inspection will also be used to assess physical stability. A comparative study across these platforms will provide insight into which strategies offer the most promise.
5. Further assessment of solubility, stability and pharmacokinetics
To support oral delivery, studies investigating the stability of the formulation and active ingredient against gastric acid, bile salts and pancreatic lipases (enzymatic degradation) in the gastrointestinal tract will be conducted in vitro. The formulation stability and release mechanisms for each formulation type could be assessed by particle size, HPLC and sub-visible/visible particle assessment. Further solubility experiments, for example using Sirius T3 or alternative, would explore how solubility changes across GI tract conditions, such as pH or media (SGF/FeSSIF) switch. In vivo pharmacokinetics can be used to determine oral bioavailability.
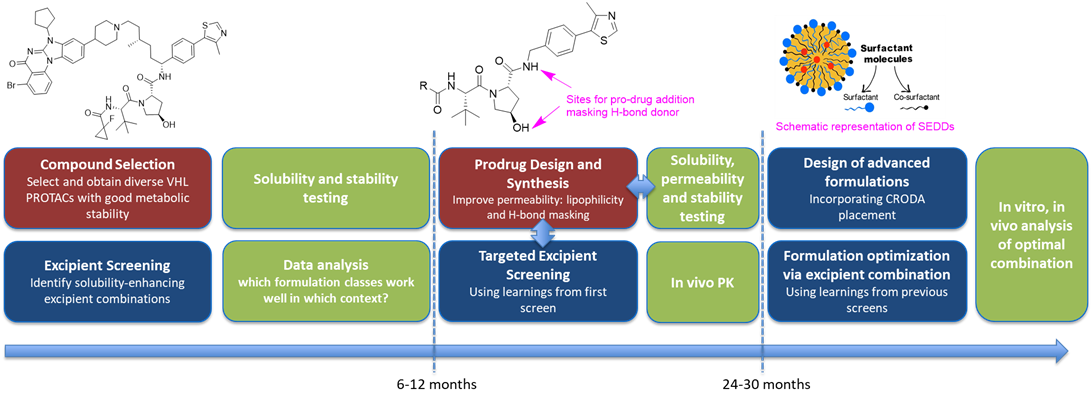
Figure 1: Project overview schematic and estimated timelines
Deliverables
This project will build a practical understanding of how formulation approaches can be applied to improve the solubility and permeability of VHL-based PROTACs. By screening a diverse set of compounds and formulations, we will define which strategies are most likely to enhance solubility without introducing chemical instability, across a range of physicochemical profiles. This will allow us to design targeted formulation screens that can be applied early in optimisation—even when only small amounts of material are available—supporting more reliable preclinical experiments and faster progression to proof-of-concept.
We will also explore prodrug strategies to improve permeability, supported by in vitro and in vivo data. By incorporating an understanding of what physicochemical properties can be solubilised through formulation in our compound design cycles, we aim to develop PROTACs with improved absorption and hence oral bioavailability. Based on the knowledge gained, rational combinations of excipients can be designed to further optimise solubility.
We will investigate the use of advanced delivery platforms—including polymeric nanoparticles, lipid-based carriers, and SEDDS. As well as solubility enhancement, these systems can provide further advantages, such as enhancement of permeability and protection against chemical and enzymatic degradation. These systems will be evaluated for their performance under gastric conditions, including exposure to acid, bile salts, and digestive enzymes, to assess suitability for oral delivery.
Together, these studies will provide a toolkit for selecting and developing formulations that are compatible with PROTACs and suitable for progression into preclinical development.
Learning objectives
The student will gain valuable expertise in interdisciplinary research, including hands-on experience of compound design, synthesis, formulation and testing, with one-to-one training from experienced scientists. They will benefit from exposure to a range of drug discovery science, as well as formulation research through Croda. Our in-house training programmes provide a broad grounding in drug discovery. Students are expected to publish, and will be supported to do so. Past students in our groups have readily found employment in academia or the pharmaceutical industry.
More broadly, this project will enable mutually beneficial knowledge-sharing between ICR and Croda, bringing formulation expertise into ICR and sharing PROTAC drug discovery challenges with Croda.
BSc, MSc or equivalent in a relevant subject area, such as chemistry, biochemistry or chemical engineering. Some experience of chemical synthesis or formulation approaches is preferred.
Hornberger, Keith R., and Erika M. V. Araujo. “Physicochemical Property Determinants of Oral Absorption for PROTAC Protein Degraders.” Journal of Medicinal Chemistry 66, no. 12 (2023): 8281–87. https://doi.org/10.1021/acs.jmedchem.3c00740.
Brown, Dean G., and Hyejin Park. “Current and Emerging Prodrug Strategies.” Journal of Medicinal Chemistry 68, no. 12 (2025): 12369–91. https://doi.org/10.1021/acs.jmedchem.5c00826.
Leconte, Georges A., Gillian E. Gadbois, Yadira Sepulveda, et al. “A Lipid Prodrug Strategy Enhances Targeted Protein Degrader CNS Pharmacokinetics.” Journal of Medicinal Chemistry 68, no. 16 (2025): 17339–49. https://doi.org/10.1021/acs.jmedchem.5c00866.
Srivastava, Abhishek, Andy Pike, Magda Swedrowska, Samuel Nash, and Ken Grime. “In Vitro ADME Profiling of PROTACs: Successes, Challenges, and Lessons Learned from Analysis of Clinical PROTACs from a Diverse Physicochemical Space.” Journal of Medicinal Chemistry 68, no. 9 (2025): 9584–93. https://doi.org/10.1021/acs.jmedchem.5c00358.
Saraswat, A.L., Vartak, R., Hegazy, R., Patel, A. and Patel, K. (2022). Drug delivery challenges and formulation aspects of proteolysis targeting chimera (PROTACs). Drug Discovery Today, p.103387
Syahputra, E.W., Lee, H., Cho, H., Park, H.J., Park, K.-S. and Hwang, D. (2025). PROTAC Delivery Strategies for Overcoming Physicochemical Properties and Physiological Barriers in Targeted Protein Degradation. Pharmaceutics, 17(4), p. 501
Postges, F., Kayser, K., Appelhaus, J., Monschke, M., Gutschow, M., Steinebach, C. and Wagner, K.G. (2023). Solubility Enhanced Formulation Approaches to Overcome Oral Delivery Obstacles of PROTACs. Pharmaceutics, 15(1), p.156.
Ickenstein, L.M. and Garidel, P. (2019). Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opinion on Drug Delivery, 16(11), pp. 1205-1226