Clinical Pharmacodynamic Biomarker Group
Professor Banerji's group focuses on the development and validation of quantifiable and reproducible robust pharmacodynamic biomarkers in normal and tumour tissue that can be used in first-in-human studies.
Research, projects and publications in this group
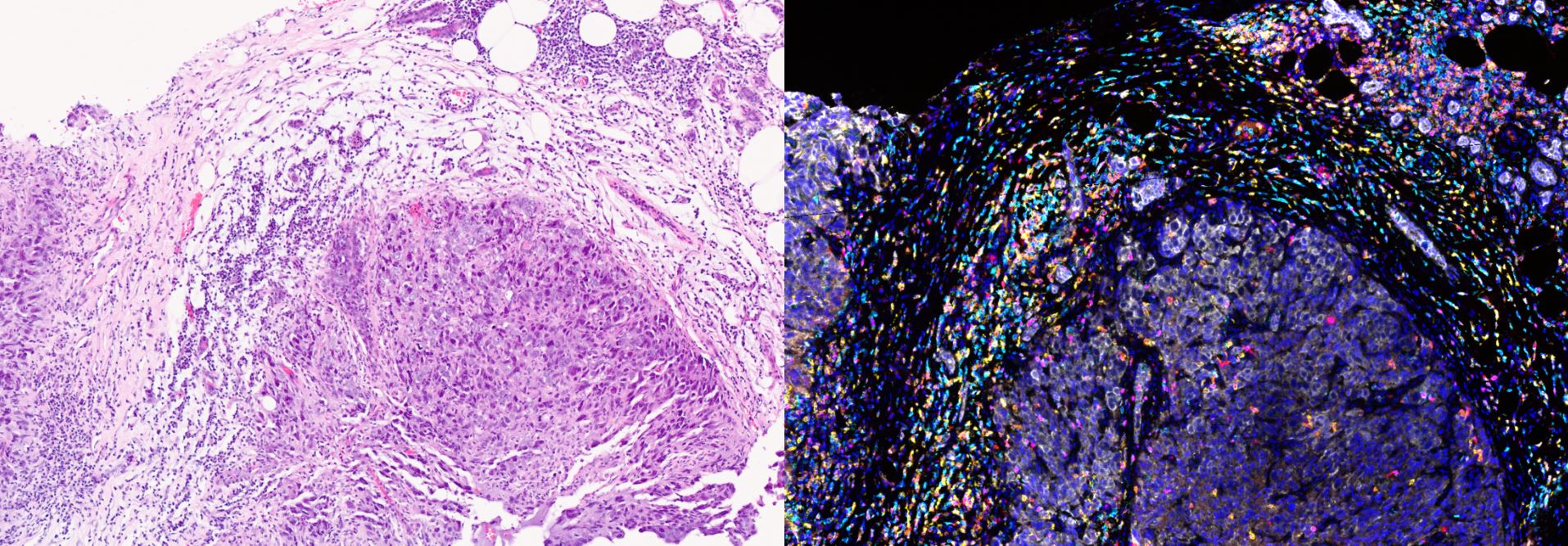
Our group focuses on the development and validation of quantifiable and reproducible robust pharmacodynamic biomarkers via methods involving MSD, Luminex, Nanostring and immunofluorescence.
The Clinical Pharmacodynamics Biomarker Group already has validated and has functioning assays of key nodes in the PI3K signalling pathway for drugs which target this pathway. It is now developing, validating and running assays focusing on DNA damage repair, such as p-CHK1 and gamma H2AX and also related to the cell cycle such as p-RB and p-HH3. In addition to these, the group has a wider focus of assays to study drugs targeting the alpha folate receptor such as shed circulating alpha folate receptor and cytokine assays for immunotherapy studies.
Professor Udai's group uses a range of platforms that measure proteins including MSD, Luminex, Nanostring and immunofluorescence. Examples of assays developed and used in clinical trials include p-AKT, p-GSK3B in platelet-rich plasma, p-PRAS40 in hair follicles, p-CHK1 in peripheral blood mononuclear cells and gamma H2AX in hair follicles by immunofluorescence. In addition, these tests are optimised for tumour tissue and have been used in multiple phase I clinical trials.
The group validates these assays to a high standard, with special attention to dynamic ranges and reproducibility of assays in addition to the stability of samples. The team has an extensive list of internal standard operating procedures and runs quality control checks as per Good Clinical Practice.