FAST-Forward Boost
FAST-Forward Boost investigates whether radiotherapy to treat breast cancer can be given in fewer treatment visits.
Status: Enrolling participants
Trial documents (password protected)
What is this study about?
FAST-Forward Boost investigates whether radiotherapy to treat breast cancer can be given in fewer treatment visits.
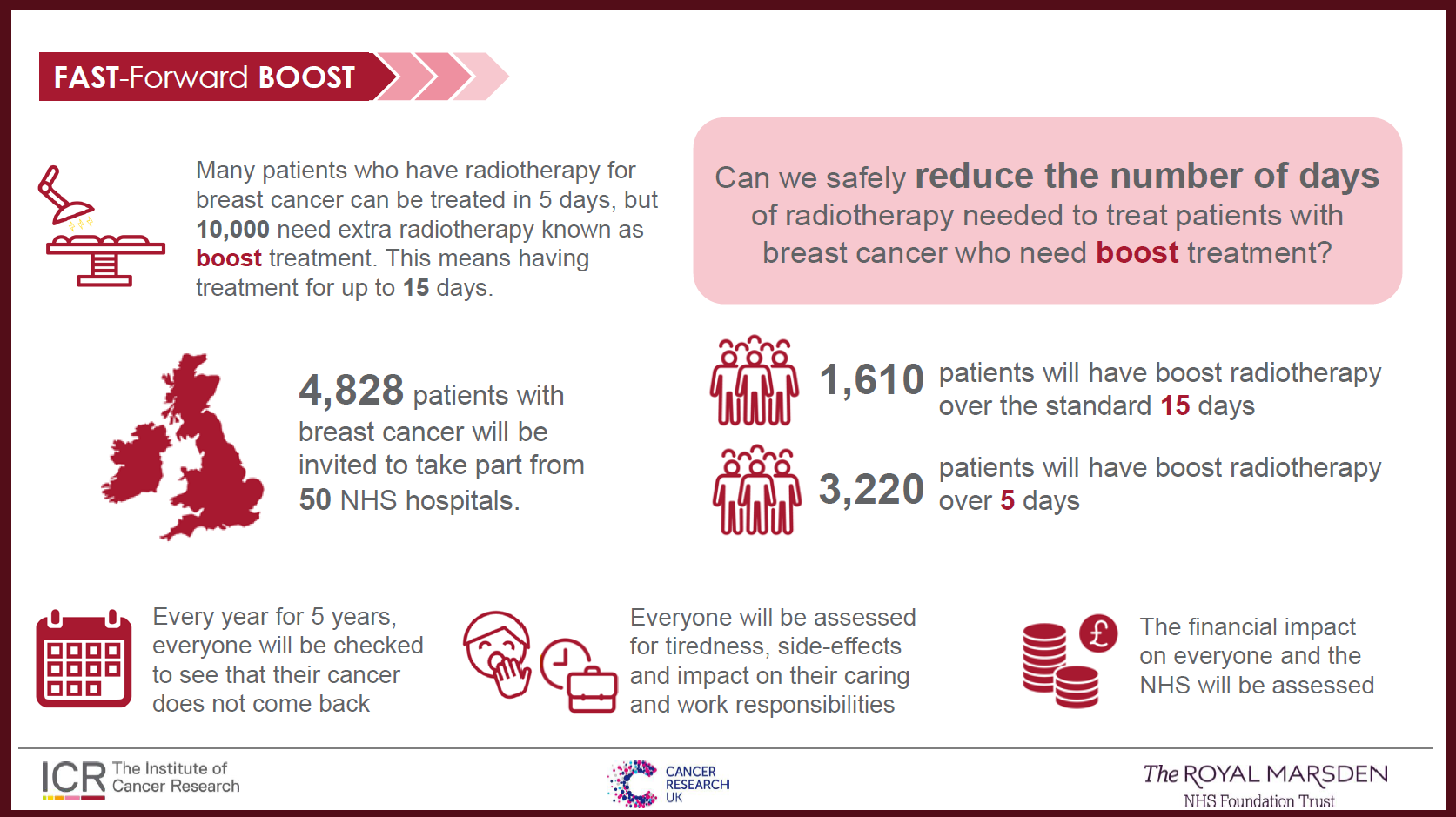
Radiotherapy is the use of radiation to treat cancer. Radiation is given after surgery to remove breast cancer. Some people with breast cancer need to have an extra dose of radiation treatment to where the cancer was removed from. This is called a boost dose.
This boost dose is usually given as part of breast radiotherapy over 15 daily treatments. The FAST-Forward Boost study will find out if boost treatment can be given as part of breast radiotherapy over 5 days. The study will check that the shorter treatment is similar to the longer treatment in preventing the cancer returning. It will also compare the side effects of the treatments.
The results of FAST-Forward Boost will be used to improve radiotherapy for people with breast cancer in the future.
Who is included in this study?
4,830 people with breast cancer who need a radiotherapy boost as part of their treatment will be included in FAST-Forward Boost. People will be invited to join the study by their medical teams at around 40 hospitals across the UK.
What are the study treatments/What does the study involve?
Everyone who joins FAST FORWARD Boost will have already had surgery to remove their cancer. After joining they will be allocated to have one of 3 radiotherapy treatments:
- Standard treatment: 15 days of radiotherapy with boost
- 5 days of radiotherapy with boost
- 5 days of radiotherapy with a slightly different boost dose
Participants will have regular check-ups during and after their treatment, and we will collect information about how they are getting on until the study is completed. The first 345 participants to be included in the study will have some additional check-ups for possible side effects during the first few weeks (this is called the Early Side-Effects Sub-Study).
Further information for participants
Our team are unable to offer any medical advice. If you are interested in finding out more about the trial or the treatments involved, please talk to your hospital doctor or GP about whether they may be a suitable option for you.
Further information for healthcare professionals
Contact details and regulatory information
Chief investigator: Dr Anna Kirby, The Royal Marsden NHS Foundation Trust
ICR-CTSU Scientific Lead: Professor Judith Bliss
Sponsor: The Institute of Cancer Research
Funding: National Institute for Health and Care Research Health Technology Assessment (NIHR157800)
Trial identifiers
REC reference: 24/LO/0910
CPMS ID: 57885
ISRCTN: 13849110
Publications and presentations
A.M. Kirby, F.H. Cafferty, K. Poole, et al. Testing a 5-fraction Simultaneous Integrated Boost in Radiotherapy for Breast Cancer: The UK FAST-Forward Boost Trial Opens to Recruitment. Clin Oncol (R Coll Radiol). 2025 Sep;45:103908. https://doi.org/10.1016/j.clon.2025.103908