A new study unveils a fast, low-cost solution for harvesting organoids – miniature three-dimensional (3D) models of human tissue – that could accelerate cancer research and make advanced cell culture techniques more widely accessible.
Researchers at The Institute of Cancer Research, London, developed the alcohol-based, enzyme-free solution, which they have called the Solution for Harvesting Organoids Efficiently (SHOE). Unlike currently used methods, which require 30–60 minutes of cold incubation, it allows researchers to recover organoids from their growth solutions in under five minutes at room temperature.
The study, published in BioTechniques, demonstrates that SHOE not only speeds up laboratory workflows but also preserves the structural integrity and growth potential of organoids in subsequent experiments.
The researchers were supported by grants from the Biomedical Research Centre, the Cancer Research UK Convergence Science Centre, the Swiss National Science Foundation and the Experimental Cancer Medicine Centre initiative.
What are organoids?
Organoids are 3D human tissue cultures that are usually developed from tumour or fluid samples from cancer patients and effectively mimic the structure and function of organs in a miniature, simplified form.
They are increasingly used in cancer research because they replicate the architecture and behaviour of tumours better than traditional 2D cell cultures – simpler, single-layer models that are easy to grow and scale but lack the structural and cellular complexity of tissues.
Organoids are grown in semi-solid solutions – similar to glue in consistency – that mimic the extracellular environment of human tissue, allowing cells to form spherical structures that more closely resemble those found in the body.
A step forward for 3D cancer models
While advantageous, harvesting organoids for experiments – such as drug testing or molecular profiling – remains a technical, financial and complex challenge that requires ethical approval and patient consent. The tissue must be kept alive, transported quickly and processed immediately, as the initial growth phase is critical and unpredictable.
Existing methods often rely on enzyme-based solutions that can damage cell surface proteins, or enzyme-free alternatives that cost more, require long incubations at low temperatures and are slower.
SHOE addresses both issues. It is non-enzymatic, reducing the risk of stripping proteins from the surface of cancer cells, and its alcohol-based formulation enables rapid organoid recovery at room temperature – making it easier to integrate into busy laboratory workflows without the need for lengthy cold incubation.
Tried and tested
The research team, led by Professor Udai Banerji, senior author of the study and Co-Director of the joint Drug Development Unit at The Institute of Cancer Research (ICR) and The Royal Marsden NHS Foundation Trust, tested SHOE on two commercial cell lines – laboratory grown cells that have been standardised and widely used in research – and two patient-derived ovarian cancer organoids. In both cases, SHOE outperformed existing commercial harvesting solutions in terms of cell recovery, while maintaining cell health and structure.
Importantly, SHOE worked across a range of solution types, including animal-based solutions and synthetic solutions. This versatility makes it suitable for a wide variety of experimental setups.
The study also showed that organoids harvested with SHOE could be successfully transferred into fresh solutions. Here, they continued growing, retaining their ability to expand and respond to treatments. This is critical for laboratories that rely on organoids for longitudinal studies or high-throughput screening.
Wider access to organoid research
The development of SHOE comes at a time when cancer research is increasingly turning to 3D models to better understand tumour biology, identify new drug targets and understand resistance mechanisms. Organoids derived from patient biopsies can preserve the genetic characteristics of the original tumour, offering a powerful tool for personalised medicine.
However, the cost and complexity of organoid culture have limited its use to well-funded laboratories and large-scale collaborations. By simplifying one of the most time-consuming steps in the organoid workflow, SHOE could help democratise access to these models. As the solution is freely available with no patent restrictions, any laboratory can adopt it without being subject to licensing fees.
Lead author Dr Jimmy Maillard, who developed the solution during his postdoctoral fellowship at the ICR, said: “The results show real promise for expanding organoid use. We’ve shown that SHOE works across different cell lines and patient-derived models, and that it preserves the ability of organoids to grow and respond to treatment. It’s a big step forward for laboratories working with 3D cultures.”
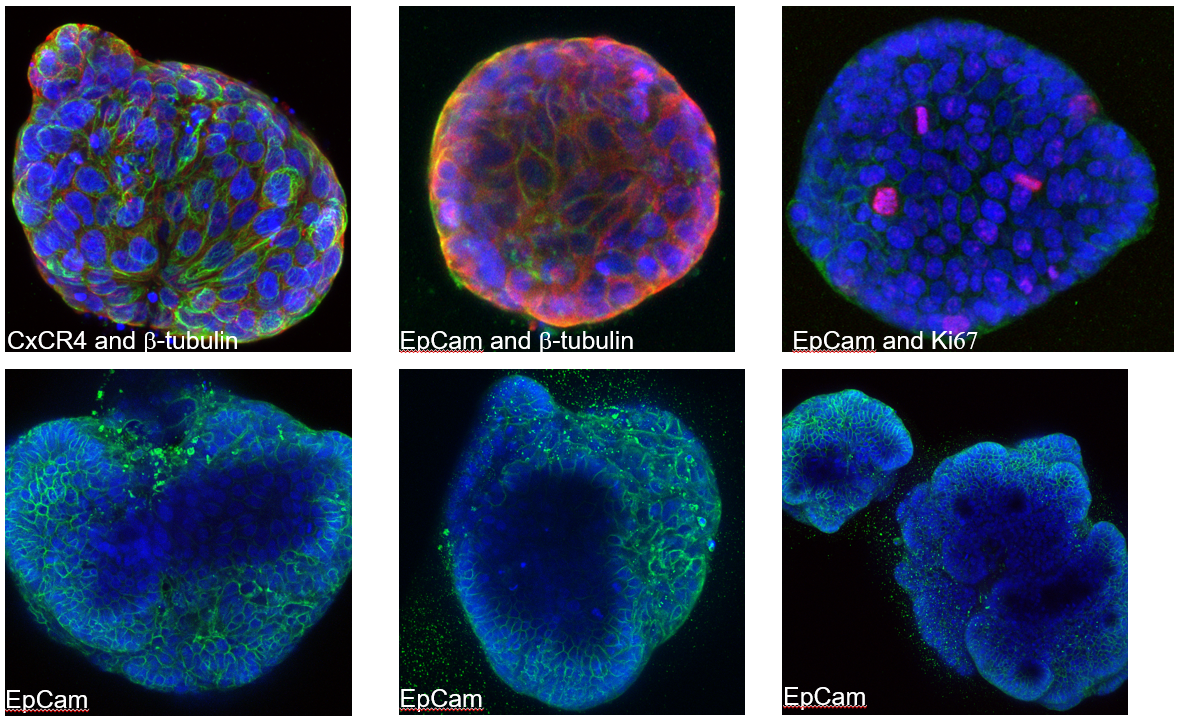
Image: Six confocal microscopy images of colorectal cancer organoids under immunofluroscence.
Looking ahead
The research team is now investigating how SHOE performs across different tumour types and solution compositions, and whether it can support more advanced applications such as proteomic and transcriptomic profiling – techniques that analyse the proteins and gene activity inside cells to better understand how they behave and respond to treatment. These techniques require large numbers of cells and intact protein structures, both of which SHOE supports.
Professor Banerji said: “With the ICR generating organoids across pancreatic, breast, lung, colorectal and ovarian cancers, the institute continues to be seen as a leader in organoid harvesting.
“We’re not claiming this is the final answer or revolutionary, but it’s a small, yet important, part of the organoid workflow – they are the future of cancer research. If we can reduce the time and cost of organoid work, we can open the door to more discoveries and better treatments.”
.jpeg?sfvrsn=2b0f927_1)