Patients and public
Patients and members of the public are vital to our research. Without patients choosing to taking part in our clinical trials our research would not be possible. We also have patients advising us on our research, providing our researchers with valuable insights about their lived experience.
What is a clinical trial?
A clinical trial checks whether new treatments are safe and effective. People taking part in trials should be representative of those affected by cancer in the wider population. Clinical trials should be designed so that anyone can join them, as long as it is safe for them to do so.
Taking part in a clinical trial
Thousands of people with cancer are currently taking part in our clinical trials. Where possible, each clinical trial page includes a link to a plain English summary.
You can also find out more about getting support and taking part in clinical trials on Cancer Research UK and Macmillan Cancer Support websites.
What are patient advocates?
A patient or public advocate helps makes sure that patient perspectives and needs are considered. They may have had cancer themselves or be a carer or family member. They could also be a member of the public who represents people affected by cancer.
Patient and Public Involvement is vital to our research at the Clinical Trials & Statistics Unit at The Institute of Cancer Research (ICR-CTSU).
Why get involved in cancer research?
- To work with research teams to advance and improve the understanding and treatment of cancer.
- To use your lived experience of cancer treatment to improve how we do clinical trial research.
- To shape the quality of care for people with cancer in the future.
- To join the network of over 90 patients and carers who are interested in our work.
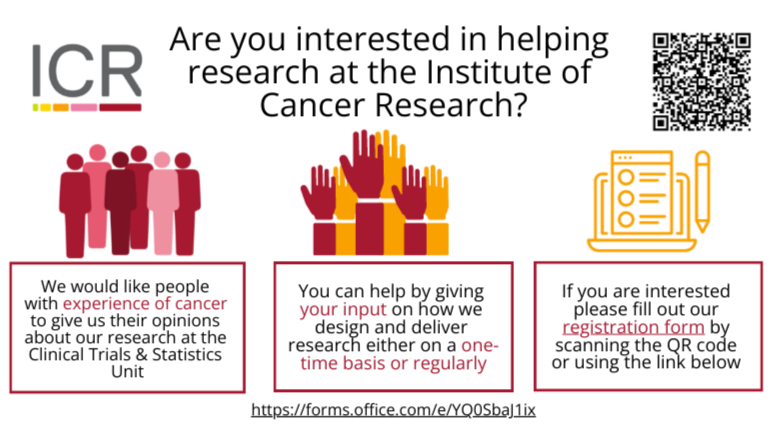
We would like your thoughts on our research
Your opinions and thoughts, whatever your background and experience, can help us to develop new or better ways to treat cancer. Your perspectives are valuable and can help design research that includes and benefits everyone who will receive future treatments. You can be involved as much or as little as you wish. For instance, you could join us for an online discussion when we are designing new research or become a patient advocate, advising us about a clinical trial over months and years. Everyone who helps us in these ways is offered reimbursement for their time and expenses.
If you are interested in getting involved, please complete this form to register your interest or email us at [email protected].
Working along organisations and community groups
To reach people who can help us with our research we work with community organisations and groups to:
- Reach people from all communities to give them the opportunity to become patient and public advocates.
- Work directly with people from community organisations or groups who can give a wider perspective on behalf of the communities they represent.
If your organisation would like to work with us in this capacity, please contact [email protected].
When and how do we involve members of the public in our research?
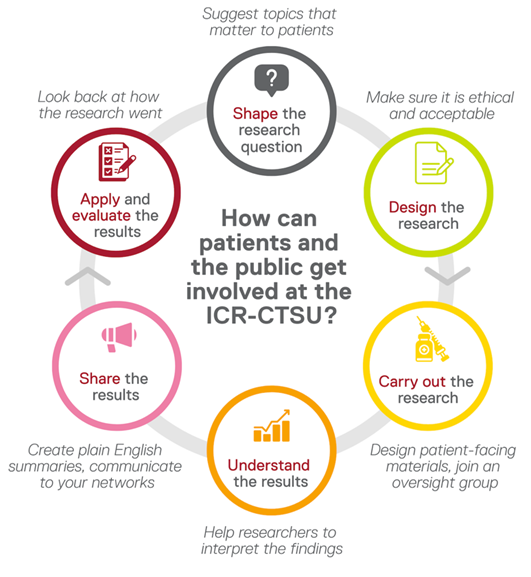
What is Patient and Public Involvement in Research?
Patient and public involvement (PPI) is where patients, carers, members of family and the community work with researchers to explore new or improved cancer treatments. PPI input is vital to our research at the ICR, as it provides researchers with valuable lived experience and opinions from a patient’s perspective.
Why get involved in research?
- To work with research teams to advance and improve the understanding and treatment of cancer.
- To add your lived experience of cancer treatment to the science of research.
- To shape the quality of care for people with cancer in the future.
- Join a network of over 50 patients and carers who regularly work us.
Some examples of how patient and public advocates have improved research at ICR-CTSU
Deciding what’s acceptable for patients
The POETIC trial was for people recently diagnosed with breast cancer. Taking part meant having multiple tumour biopsies before and after surgery.
Hospital teams were concerned that patients would find this unacceptable, as it could add stress at a difficult time.
Patient Advocates:
- Persuaded the researchers and NHS staff that people should have the choice to donate the tumour biopsies for research.
- Helped to create leaflets. These explain why the biopsies were needed and the risks involved, so that people could make their decision.
Thanks to this input, over 4,400 people joined the POETIC trial. The results showed that having the extra tumour biopsy meant many patients were able to have better, more targeted treatment.
Researchers are still learning from this valuable biopsy tissue and data collected within the trial. It also led to other trials adopting a similar approach.
You can read more about how patient advocates influenced this research here .
Shaping the future of research communication
Andrew, one of our patient advocates, has been working with an international team to improve how the results of early-stage clinical trials are shared. Early-stage trials involve giving a new treatment to people for the first time. Andrew worked alongside other patient advocates to develop a template to help researchers produce easy-to-understand summaries of the results of these clinical trials.
Creating videos to help explain research
Patient advocates have helped create videos that explain ICR-CTSU trials to people thinking about taking part. The videos are shared with patients and their family and friends to support the written information they receive. Patient advocates advise on the script and the look and feel of each video. They work with a professional video company to create short, easy to understand animations.
Below are links to videos about:
ICR-CTSU Patient and Public Involvement Newsletters
Latest editions
ICR-CTSU involvement opportunities
Are you interested in being in a video about your experience of radiotherapy?
The Cancer Research UK & UCL Cancer Trials Centre and the Institute of Cancer Research (ICR) teams are working together to create a series of videos for people who are considering taking part in a research study or who want to learn more about research.
As part of this project, we are looking to speak with individuals who have been involved in radiotherapy research studies, as patients or carers and who are willing to share their experiences and contribute their voices to the project.
If you are interested in getting involved, please email [email protected].
Useful resources for patient and public advocates
Patients have worked with us to develop a jargon-buster that might be useful when working with researchers.
Other relevant documents and training resources are available to our patient advocates using the following link: PPIE documents (password protected)