REDEFINE
Exploring the Potential Benefits of Decentralisation of Cancer Clinical Trials in the United Kingdom and its Acceptability, Feasibility, Facilitators and Barriers from a Diverse Stakeholder Perspective
Status: Enrolling participants
What is the study about?
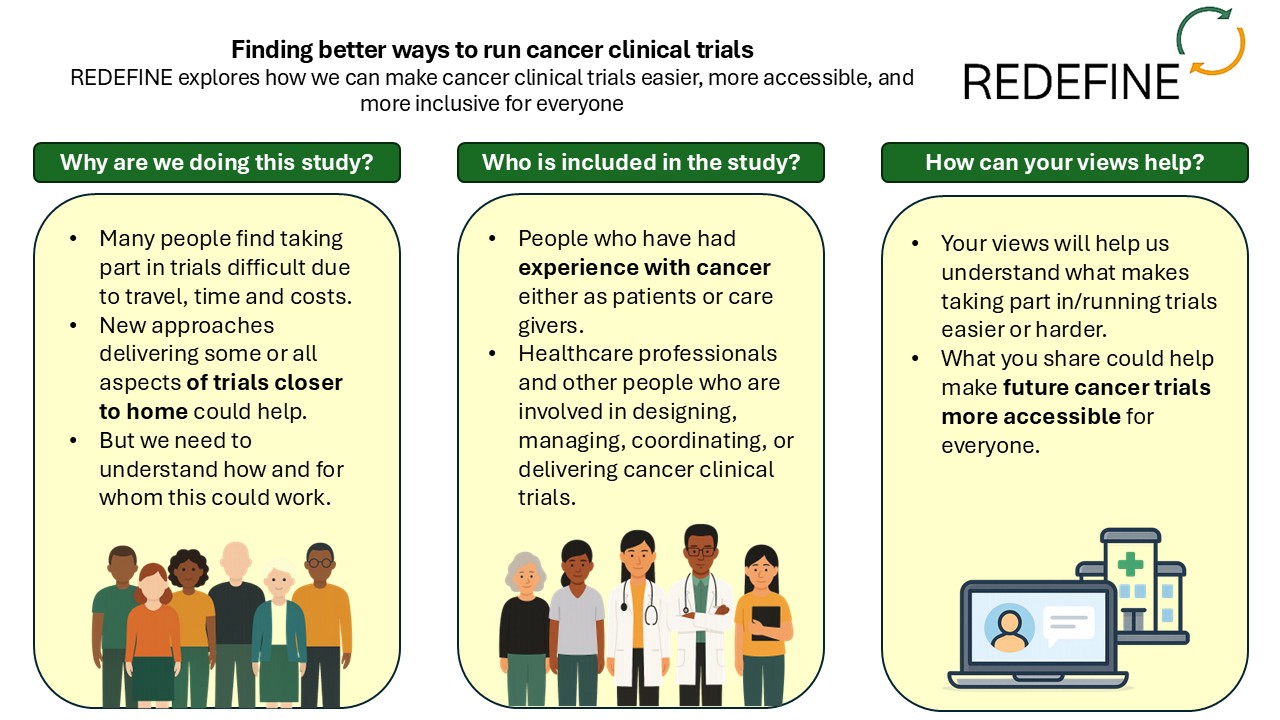
Cancer clinical trials often require extra tests and questionnaires, usually at a single NHS hospital, which can be inconvenient for patients and place extra pressure on NHS staff. Since COVID-19, healthcare has evolved to incorporate methods such as video calls and home testing kits, and clinical trials need to adapt accordingly.
Many people with cancer are unable to join trials due to travel, financial pressures, caregiving responsibilities, and other barriers. The REDEFINE study aims to identify ways to make cancer clinical trials more accessible to everyone, regardless of their circumstances.
Who is included in the study?
The study seeks adults who have recent experience with cancer diagnosis and treatment, either as patients or caregivers. We are particularly interested in hearing from people with any level of involvement in clinical trials, including those who have participated in a trial, those who have been offered and turned down trial participation, and those who have joined a trial and later decided to withdraw.
We will also be talking to healthcare professionals and other people in the UK who are involved in designing, managing, coordinating, or delivering clinical trials across any cancer types or stages.
What does the study involve?
The study has four main parts:
1. Speaking to people affected by cancer and healthcare professionals
We will interview patients, caregivers and professionals involved in clinical trials to learn about their experiences. This will help us understand the challenges people face, especially around travel, time and costs.
2. Understanding NHS readiness and co-designing solutions
We will survey staff at NHS hospitals to see how prepared they feel to use decentralised trial methods. We will then run workshops with patients and professionals to design ideas and solutions together.
3. Developing the solutions further
We will refine the ideas from the workshops and prepare a small pilot study to test whether the solutions are practical and useful in real trial settings.
4. Testing the approach in a real cancer trial
We will test the solutions in an actual clinical trial to see if it is easy to use, acceptable to patients and staff, and suitable for wider use in the future.
Further information
For HCPs, please email [email protected] if you would like to receive a copy of the protocol.
Contact details and regulatory information
Chief Investigator: Dr Fay Cafferty, The Institute of Cancer Research
Trial management contact: [email protected]
Sponsor: The Institute of Cancer Research
Trial identifiers:
IRAS ID: 358868
REC Ref: 25/NW/0309
CCR: 6235