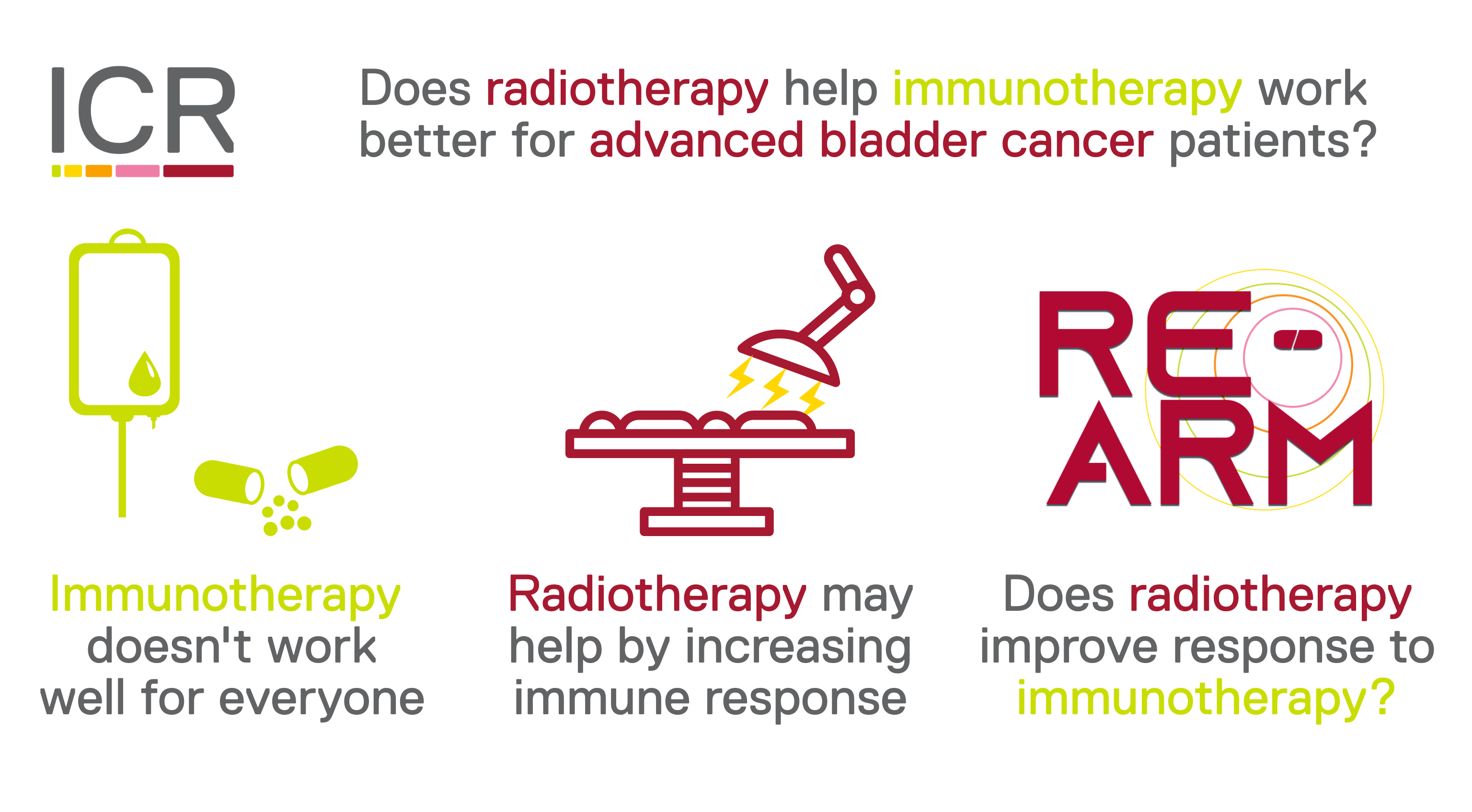
RE-ARM
RE-ARM investigates whether giving radiotherapy to people receiving treatment for advanced bladder cancer helps the treatment work better.
Disease site: Urological cancers Bladder cancer
Status: In follow-up
What is the study about?
RE-ARM investigates whether giving radiotherapy to people receiving treatment for advanced bladder cancer helps the treatment work better.
People with advanced bladder cancer which has spread around the body are often treated with atezolizumab. Atezolizumab is a type of treatment called an immunotherapy. It works by reactivating the body’s immune system to attack the cancer cells. But atezolizumab doesn’t always work to shrink peoples’ cancer straight away, and may not ever work well for some people.
Giving radiotherapy alongside atezolizumab may help kickstart peoples’ immune systems and help the atezolizumab work better to shrink their cancer.
RE-ARM is investigating whether giving a short course of radiotherapy to people who have been receiving atezolizumab, or a similar drug called avelumab, could help the immunotherapy treatment work better.
Who is included in the study?
RE-ARM includes people with advanced bladder cancer who have been treated with atezolizumab or avelumab for up to six months without their cancer shrinking. People with cancers of other parts of the urinary tract can also join the trial if their treatment has not been working. 102 people will be included in RE-ARM, from NHS hospitals across the UK.
What are the study treatments?
Everyone who joins RE-ARM, including those who were taking avelumab, receive atezolizumab after joining the trial. Participants are in two treatment groups:
- Atezolizumab treatment alone
- Atezolizumab treatment plus a short course of radiotherapy
Participants have regular check ups during and after their treatment and we collect information about how they are getting on until the study is completed.
Further information for participants
Patient Information Sheet - atezolizumab
Patient Information Sheet - avelumab
A detailed summary is available on Cancer Research UK’s website.
Trial documents (password protected)
Further information for healthcare professionals
Contact details and regulatory information
Chief Investigator: Professor Robert Huddart, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust
ICR-CTSU scientific lead: Professor Emma Hall
Trial management contact: [email protected]
Sponsor: The Institute of Cancer Research
Funding: Cancer Research UK (CRUKE/19/009) and Roche Products Ltd
Trial identifiers
EUDRACT number: 2020-004893-23
REC reference: 21/LO/0150
CPMS ID: 48346
Publications and presentations
A. Wilkins, E. Hall, R. Lewis, H. Gribble, A. Melcher, R. Huddart, RE-ARMing the Immune Response to Bladder Cancer with Radiotherapy, Clinical Oncology, 2022
R. Huddart, R. Lewis, S. Brown, E. Cheadle, A. Choudhury, S. Crabb, C. Emery, H. Gribble, J. Haviland, T. Illidge, M. Linch, H. Scowcroft, A. Sohaib, I. Syndikus, R. Walshaw, A. Wilkins, A. Melcher, E. Hall, RE-ARM – a multicentre phase II randomised controlled trial of radiotherapy plus atezolizumab in metastatic urothelial carcinoma – investigating the abscopal effect. Presented at: National Cancer Research Institute Cancer Conference; 2021 Nov 8-12