A new study suggests that silencing CXCL12, a gene involved in tissue scarring and repair, could help reduce the formation of scar tissue that can caused by radiotherapy. Ultimately, the researchers hope this approach could improve treatment outcomes for breast cancer patients undergoing radiotherapy and reconstructive surgery.
Researchers at The Institute of Cancer Research, London, investigated in rats whether silencing CXCL12 could prevent radiation-induced fibrosis (RIF), improve the treatment of tumours surrounded by dense scar tissue and enhance the effectiveness of systemic therapies, such as chemotherapy and immunotherapy.
There is a clinical imperative in preventing this scarring as it can severely impact cancer survivors’ quality of life due to it causing chronic pain, limited mobility and leading to cosmetic deformities. Additionally, RIF can increase complication rates in breast reconstruction and cause delays in the patient’s recovery journey, hindering their physical and emotional wellbeing.
This study, published in Molecular Cancer Therapeutics, was primarily funded by the Wellcome Trust through a training fellowship for James T Paget, first author of the study and US-based plastic surgeon with expertise in breast reconstruction after cancer. Additional financial support was provided from the NIHR Biomedical Research at The Royal Marsden NHS Foundation Trust and the ICR.
A regulator of fibrotic states
RIF is a common side effect of radiotherapy, resulting from tissue damage caused by radiation exposure. It is characterised by inflammation, scar tissue formation and an imbalance in the body’s immunological processes. CXCL12 has been identified as a regulator of fibrotic states – such as cirrhosis and pulmonary fibrosis – with studies suggesting it may also play a role in the development and progression of RIF. However, its role in RIF and its potential as a therapeutic target have not been analysed in detail prior to this study.
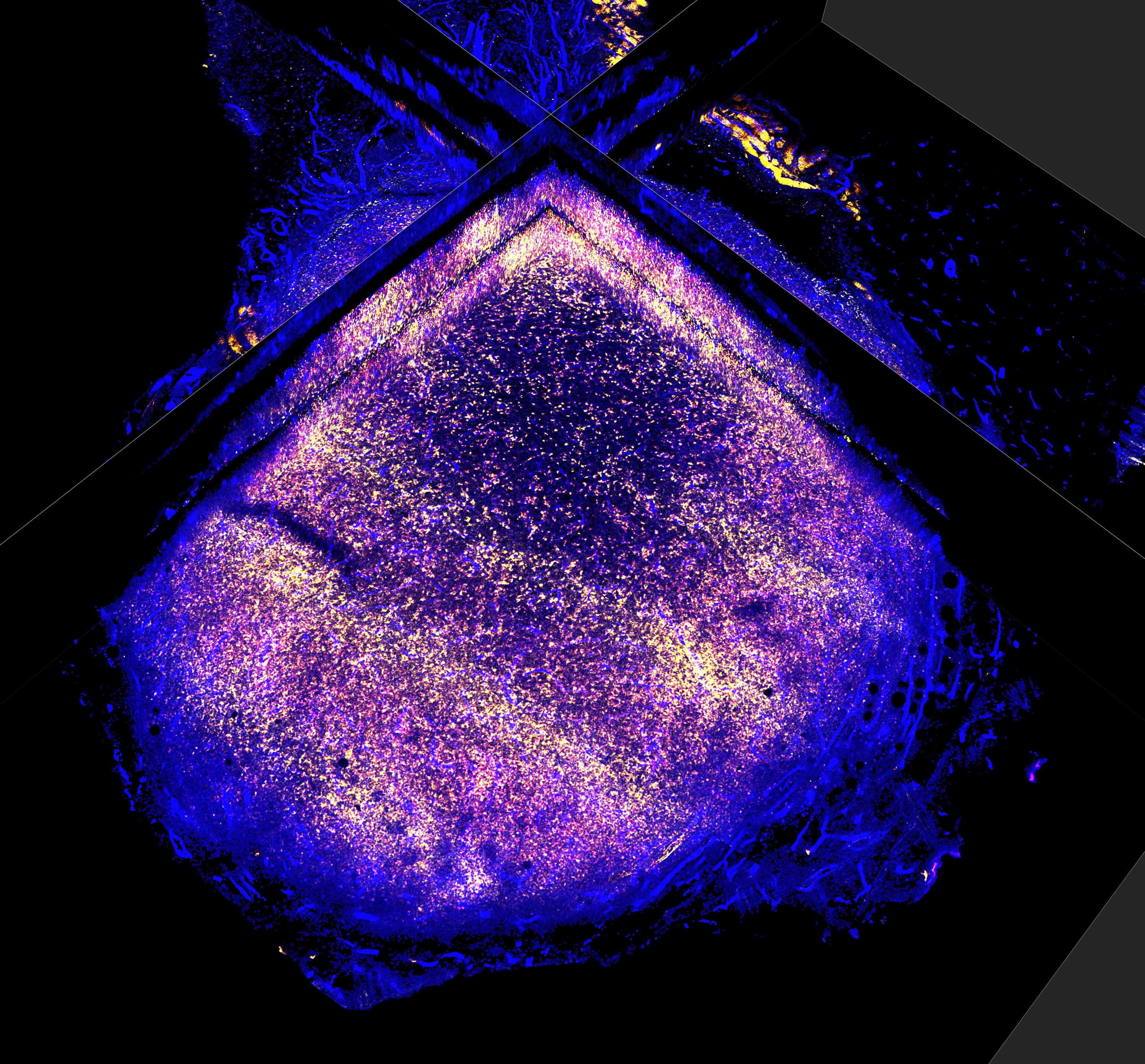
The study used a pre-clinical mouse model to examine how silencing CXCL12 in vivo affects RIF. For this, skin flaps were surgically modified and exposed to radiation, with gene therapy delivered into the rats skin flap cells via lentiviral vectors to either silence or overexpress CXCL12 expression before irradiation.
Though this use of gene therapy served as a powerful tool in the rat model used for this study, this approach is not currently used in clinical settings. The researchers suggest that in future human applications, pharmacological inhibitors targeting the CXCL12 pathway could offer a more practical and translatable alternative.
Utilising molecular and imaging techniques, such as RNA sequencing and flow cytometry, the effects were reviewed and further validated by analysing human tissue samples from breast cancer patients who underwent radiotherapy to compare fibrosis-related markers against the animal model.
Silencing CXCL12 yields antifibrotic benefits
The research team found that CXCL12 is a key mediator of the immune response to radiotherapy. Their findings confirmed that silencing this gene is associated with the preservation of normal tissues after radiotherapy and made tumours more responsive to radiotherapy.
When tumours were implanted in these tissues, the researchers observed the formation of a fibrotic capsule around them – a thick layer of connective tissue acting as a barrier to immune cells. In normal, untreated tissues, this capsule is typically thicker, which can block the infiltration of key immune cells, particularly T cells. However, in tissues where CXCL12 was silenced, the fibrotic barrier was significantly thinner – reduced by approximately 50 per cent – allowing for a two point five increase and greater diversity of effective T cells to enter the tumour. This suggests that CXCL12-targeted therapy reduces fibrosis, making the tumour environment more accessible to immune cells and enhancing the effects of radiotherapy for better tumour control.
The role of CXCL12
Although the study provides new evidence that CXCL12 expression increases in tissues following exposure to radiotherapy – which supports the relevance of the preclinical model – the research team did not formally determine its exact origin.
The role of CXCL12 remains unclear, with challenges involved in the translation of this concept. While transporting tissue from one part of the body to another – known as free tissue transfer – the study was the first to use it for gene-targeted therapy. With the existing animal model, they had shown for the first time that it’s possible to construct these tissue grafts – also called free flaps – using viral gene therapies, which hasn’t been described previously.
Senior author of the study Dr Aadil Khan, Consultant Plastic, Reconstructive and Aesthetic Surgeon at The Royal Marsden and an Honorary Appointment in the Targeted Therapy Group at the ICR, said:
“We know radiotherapy is effective, but some tumours are more resistant than others. This study suggests that targeting CXCL12 could reduce side effects while making radiotherapy more effective, particularly for treatment-resistant tumours.
“It may be that by using a CXCL12-directed therapy in this way, we can reduce the side effects of radiotherapy while enhancing its effectiveness – particularly in tumours surrounded by dense fibrous tissue that typically do not respond well to chemotherapy or immunotherapy.”
Co-author of the study Professor Kevin Harrington, Professor of Biological Cancer Therapies at the ICR and a consultant oncologist at The Royal Marsden, said:
“The scar tissue that can develop after radiotherapy is difficult to treat and can be a debilitating complication that leads to pain, disfigurement and loss of function. Previous studies of breast reconstruction have reported higher incidence of reconstructive, fibrosis-related complications and worse patient-reported outcomes with radiotherapy. There is a clinical imperative to prevent this scarring – known as RIF – due to its impact on quality of life during survivorship.”
While the role of CXCL12 within the tumour microenvironment remains unclear, previous analyses identifies a correlative relationship between prognosis and CXCL12 expression across a variety of cancers.
A promising therapeutic target
This research highlights CXCL12 as a promising therapeutic target, one that can enhance radiotherapy’s effectiveness against cancer but can also minimise its side effects, ultimately improving quality of life for patients.
Dr Aadil Khan added:
“There is a further piece of work to be done on whether the same approach could make tumours more permeable to systemic therapies as well – something we did not evaluate in this study. However, I think that as a combination therapy, there is the concept of a ‘win-win’ therapy – something that can both enhance the effectiveness of radiotherapy and reduce its side effects.”