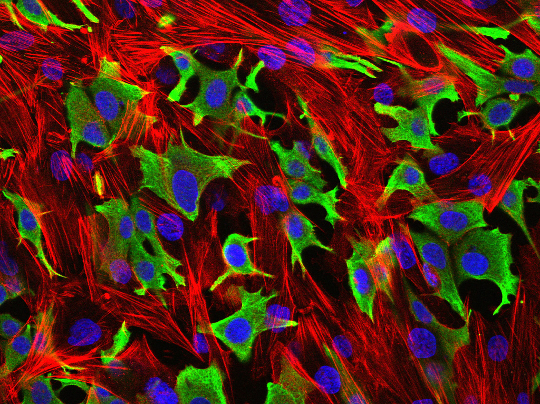
Breast cancer cells (green) invading through a layer of fibroblasts (red). (Luke Henry / the ICR)
Scientists have identified a molecule crucial to the growth of triple-negative breast cancers that they believe could now be targeted by drugs to help treat patients resistant to chemotherapy.
In a new study — an innovative collaboration between Breast Cancer Now’s Research Unit at King’s College London and the charity’s Toby Robins Research Centre at The Institute of Cancer Research, London — published today in the journal Nature Medicine, scientists have identified the role of a molecule called PIM1 in driving and controlling triple-negative breast cancers, influencing the ‘death threshold’ of cancer cells in the face of treatments such as chemotherapy. The study was funded by Breast Cancer Now.
Around 15% of all breast cancers are ‘triple negative’, with around 7,500 women in the UK being diagnosed each year. More common among younger women, triple-negative breast cancers can sometimes be more aggressive than other types of breast cancer and often have a poor prognosis.
‘Triple-negative’ means that they lack the three receptors which are normally used to classify breast cancers: the oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). This form of breast cancer therefore cannot be treated with targeted drugs commonly used to interfere with these receptors, such as tamoxifen and aromatase inhibitors for ER and PR-positive breast cancer, or Herceptin for HER2-positive disease — leaving triple-negative breast cancer patients with fewer treatment options: typically chemotherapy in addition to surgery and radiotherapy.
When faced with chemotherapy, breast cancer cells suffer damage which triggers a signal inside the cell telling itself to self-destruct. This process, known as apoptosis, is essential for chemotherapy to be effective.
Hijacked molecule
However, the research teams — led by Professor Andrew Tutt at King’s College London and the ICR, and Dr Pascal Meier, also at the ICR — have discovered that the molecule PIM1 has been hijacked and is being over-produced in the majority of triple-negative breast cancers, helping them to survive by making them more resistant to these ‘death signals’.
This hijacking helps triple-negative breast cancers to survive by making them more resistant to the ‘death signals’ prompted by chemotherapy.
The finding provides some explanation as to why a significant group of triple-negative breast cancers are very aggressive and resist the effects of chemotherapy.
Triple-negative breast cancers become addicted to PIM1 to survive, whereas the molecule has little impact on the functioning of normal cells. This suggests that PIM1-inhibitors could be a targeted way of hitting triple-negative breast cancer cells and making them more sensitive to other therapies.
In particular, the scientists were able to reveal for the first time the crucial role played by PIM1 in controlling key drivers in triple negative breast cancer including:
- cell ‘death protectors’ or self-preservation molecules, such as BCL2 or MCL1
- growth accelerators, such as EPHA2 and PTPN11
- a master regulator called MYC, which is known to be involved in the growth of many cancers and is usually switched on in chemotherapy-resistant triple-negative breast cancers – but which has so far proved difficult to target directly with drugs.
These proteins are major controllers of the malignant features of triple-negative breast cancer cells, such as their insensitivity to ‘death signals’. They have previously been found to be particularly difficult to target directly with drugs but, by blocking PIM1, scientists now believe many of these mechanisms can be indirectly switched-off.
This in turn could remove some of the drive for tumour growth but also push triple-negative breast cancer cells — but not normal cells — closer to the ‘cliff-edge of death’ and enable chemotherapies used in the clinic to destroy them.
The researchers at KCL and the ICR looked at patient sample data from three independent studies from Guy’s Hospital, The Cancer Genome Atlas, and METABRIC (a total of 2,255 breast cancer patients, of which 319 were of triple-negative breast cancer patients).
They found that levels of the PIM1 gene message were increased in the majority of triple-negative breast cancers when compared with other subtypes, suggesting that a significant proportion of patients with triple-negative breast cancer could benefit from drugs that block PIM1.
Already in trials
In the lab the researchers demonstrated that genetically depleting production of PIM1 reduced the growth of triple-negative breast cancer cells. They then studied a PIM1-inhibitor drug, which blocks PIM molecules.
In cells grown in the lab, the drug had a similar effect to genetically depleting the production of PIM1, stopping breast cancer growth — and when they treated mice that were carrying tumours formed from triple-negative breast cancer cell lines or from patients with triple-negative breast cancer, the PIM1-inhibitor slowed tumour growth.
Combining the PIM1-inhibitor drug with chemotherapy drugs then improved their effectiveness in stopping the growth and increasing apoptotic death of triple-negative breast cancer tumours cells.
In particular, treating both triple-negative breast cancer cells lines and human tumour samples implanted into mice with the PIM1-inhibitor and eribulin (an approved and commonly used breast cancer chemotherapy drug) proved to be more effective in reducing the size of tumours than using chemotherapy alone. This is a treatment strategy that the scientists hope may prove promising in future clinical trials.
Crucially, not only are PIM1-inhibitors already in development and being tested in clinical trials for blood cancers, but results from the trials suggest that they could be well-tolerated by patients.
Research published simultaneously in Nature Medicine today from a group led by Dr Andrei Goga from the University of California, San Francisco also confirms the role of PIM1 in triple-negative breast cancer.
That these two complementary studies use different approaches in independent experiments but arrive at similar conclusions increases the body of evidence that supports the further investigation of PIM1-inhibitors in treating triple-negative breast cancer.
Professor Tutt’s team’s next steps will be to work with companies that have developed PIM1-inhibitors to design and conduct clinical trials in women with triple-negative breast cancer – something the authors believe could happen within two years.
A 'hugely exciting' advance
Professor Tutt, Director of the Breast Cancer Now Toby Robins Research Centre at the ICR and the Breast Cancer Now Research Unit at King's College London, and a Consultant Oncologist at Guy's and St Thomas' NHS Foundation Trust, said: “Many triple negative breast cancers are very resistant to chemotherapy and are ‘driven’ by genes that are very difficult to target with drugs.
"We have shown that PIM1, a molecule for which drugs are already in trials in other diseases, is so important for what makes a triple-negative breast cancer cell malignant that they become addicted to it. PIM1 also rescues them from the cliff-edge of death caused by chemotherapy by controlling these ‘driver’ and ‘death protection’ genes — genes that are themselves difficult to drug.”
“It is early days but as PIM1-inhibitor drugs have already been discovered they may give us a new way to hit these cancer genes. The hope would be that these drugs could strip triple-negative breast cancers of their defences so that they can be pushed over the cliff by other breast cancer treatments.”
Baroness Delyth Morgan, Chief Executive at Breast Cancer Now, said: “This is a hugely exciting advance for an important group of patients in desperate need of more treatment options.
“Triple negative breast cancer is often aggressive and more common in younger women, and chemotherapy drugs remain the only option for these women. While these work very well for some, if patients’ cancers become resistant there are few other options.
“A new targeted treatment for triple negative patients would be a major breakthrough. Drugs that inhibit the action of crucial molecule PIM1 are in clinical trials for leukaemia already, and look like they could be well-tolerated, and this gives us real hope of a new option in the future.
“Fundamentally, this finding demonstrates the importance of continued innovation and collaboration in trying to answer the biggest questions remaining in breast cancer.”