
COBRA
COBRA investigates whether a different type of treatment could be a good option for people with early bladder cancer.
Status: In set up
Trial Documents (password protected)
COBRA investigates whether a different type of treatment could be a good option for people with early bladder cancer.
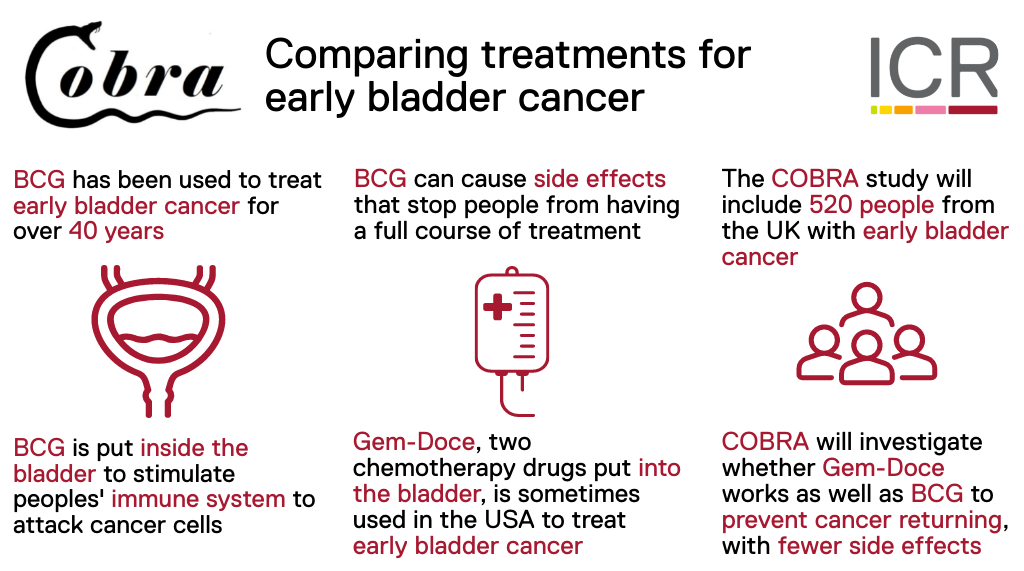
Early bladder cancer can have a high risk of returning after surgery. People with a high risk of their cancer returning are given a course of BCG treatment, put directly into their bladder regularly for up to 3 years. People having BCG treatment can have a lot of side effects which can stop them completing a full course of treatment.
COBRA investigates whether giving people a different type of treatment into the bladder, using existing chemotherapy drugs, works as well as BCG without causing as many side effects. Results will be used to improve treatment for people with bladder cancer in future.
Who is included in the study?
COBRA includes people with early bladder cancer. Everyone who joins the trial will have had surgery to remove their cancer. If surgical samples show their cancer has high risk of returning, and they would normally receive BCG as a further treatment, they may join the trial. Up to 520 people will be included from NHS hospitals across the UK.
What are the study treatments?
Everyone who joins COBRA will be in one of two treatment groups:
- Standard BCG treatment given into the bladder regularly over two years
- Gem-Doce treatment given into the bladder regularly over two years
Participants will have regular check-ups during and after their treatment and we will collect information about how they are getting on until the study is completed.
Further information for participants
Patient information sheet
A detailed summary is available on Cancer Research UK’s website.
Our team are unable to offer any medical advice. If you are interested in finding out more about the trial or the treatments involved, please talk to your hospital doctor or GP about whether they may be a suitable option for you.
Further information for healthcare professionals
Protocol
Contact details and regulatory information
Chief Investigator:Professor Rakesh Heer, Imperial College London
Study Chair: Mr Wei Shen Tan, Yale School of Medicine
ICR-CTSU methodology lead: Professor Emma Hall
Trial management contact: [email protected]
Sponsor: The Institute of Cancer Research
Funding: National Institute for Health and Care Research: Health Technology Assessment NIHR160459
Trial identifiers
REC reference: 25/SC/0262
CPMS ID: 57987
ISRCTN registry: ISRCTN92816346
Publications and presentations
There have been no presentations or publications to date.

