Clinical Pharmacology Adaptive Therapy Group
Professor Banerji's group aims to study re-wiring of signal transduction to understand and overcome mechanisms of drug resistance and, in addition, to understand exploit cancer evolution using pharmacological tools.
We are focused on the re-wiring of signal transduction using established cell lines to control mechanisms of drug resistance and comprehend the evolution of cancer.
Rewiring of signal transduction
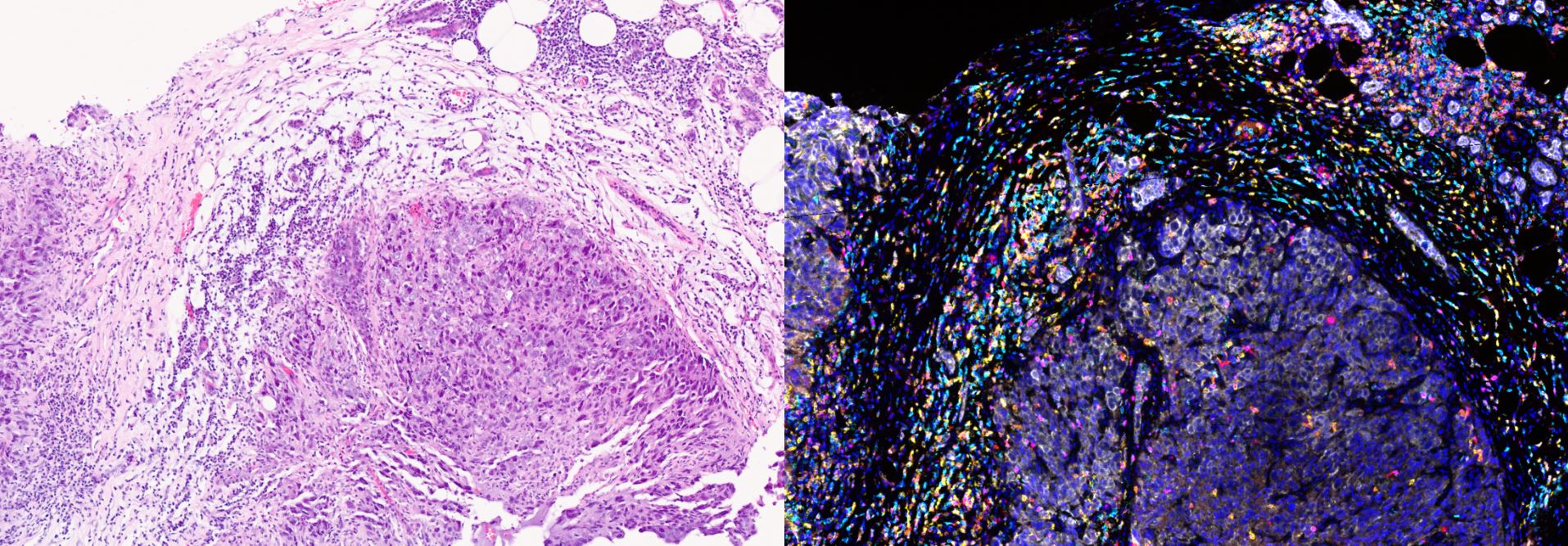
This group is working on the set-up and validation of a highly sensitive antibody-based assay on the Nanostring platform which will allow quantification of 50 - 100 phosphoproteins/proteins. It plans to digest tumour tissue obtained during surgery or from biopsies and expose them to a matrix of 20 - 30 anticancer drugs before obtaining phosphoproteomic data.
It also aims to use organoids and patient-derived xenografts to take this forward. The proteomic data will be modelled with the Computational Biology and Chemogenomics Team led by Professor Bissan Al-Lazikani. The group also supports PhD students studying the re-wiring of signal transduction in colon cancer and the role of stroma in influencing signal transduction.
The group also currently works with Professor Andrea Sottoriva at the Centre for Evolution and Cancer at the ICR to look at barcoding of cancer cells and the study of evolutionary trajectories of clones under experimental conditions. It is also studying mechanisms of resistance, collateral drug sensitivity and resistance of these emergent clones.
The primary area of this group is focused on the re-wiring of signal transduction using established cell lines as well as fresh cancer cells derived and isolated from patients and then exposing them to novel anticancer drugs. Pre- and post-proteomic profiling provides insights into mechanisms of drug resistance and how to overcome this with combination therapies. The initial work was carried out using cancer cells isolated from ascites and pleural effusions; the group in now developing expertise in organoid and patient-derived xenograft tissue.
The group develops its own antibody-based proteomic platforms and collaborates with teams involved in mass spectroscopic methods, led by Jyoti Choudhary. The group generates significant amounts of data and collaborates with the ICR's Computational Biology and Chemogenomics Team led by Professor Bissan Al-Lazikani to develop and decipher the data.
The secondary focus of this lab is the study of the pharmacological effects on cancer evolution in experimental models and methods to quantify this and herd cancer cells to a vulnerable state.
Professor Banerji works with Professor Andrea Sottoriva in the Centre for Evolution and Cancer at the ICR and plans to translate these concepts in to the clinic.
Vacancies at the ICR
Working at the ICR
Research Group Leader, ICR Clinical Trials and Statistics Unit (ICR-CTSU)
Department/Directorate Information: Division of Clinical Studies - Clinical Trials and Statistics Unit (ICR-CTSU) The ICR-CTSU is a Cancer Research UK-funded, internationally recognised methodologist led clinical trials unit, providing cancer-focused clinical trial research expertise. We lead pioneering, efficient, high-quality, and impactful trials across the phases. Our expertise ranges from experimental medicine early phase studies exploring biological efficacy to trials which may deliver widespread change to routine practice, underpinned by applied methodology to drive forward clinical trial innovation. See our clinical trials Role Summary The Group Leader will lead a component of ICR-CTSU’s portfolio of clinical trials research. The post holder will join an existing faculty and seek to further develop and grow the portfolio in line with ICR-CTSU’s overall strategy; taking responsibility for a number of ongoing trials as well as the development of new trials. There will be the opportunity to develop and grow a team including the potential for a Postdoctoral Training Fellow and/ or a Trial Manager. We seek an experienced statistician / biostatistician with a strong research interest in clinical trials methodology and a passion for direct involvement in the oversight and leadership of academic clinical trials. The successful candidate will work closely with the Director of ICR-CTSU to further enhance the Unit’s internationally recognised strength in clinical trial design, conduct and analysis. The post holder will be expected to make a substantial independent intellectual contribution to clinical trials projects and be proactive in leading and contributing to broad initiatives that enhance the overall effectiveness of ICR-CTSU. The appointee will contribute to the overall scientific life of the ICR including the newly established ICR/Royal Marsden Hospital’s Centre for Trials and Population Data Science, by providing mentorship to more junior colleagues and acting as an academic leader. We seek an individual who will work closely and collaboratively with other faculty/Group Leaders at the ICR and with international/national key opinion leaders to extend the breadth and depth of ICR-CTSU’s biologically rich clinical trials portfolio. In partnership with clinical opinion leaders, this individual will generate research funds to conduct and deliver clinical trials research at the international forefront. Presentation at national and international conferences, production of top-quality research outputs and substantial professional contribution to wider clinical trial network bodies are expected. Enthusiasm for team-based science in a collaborative interdisciplinary environment is essential. The appointment will be based on track record and the ability and willingness to engage in team science. The successful appointee will have access to ICR’s successful PhD training programme and core facilities. Key Requirements Higher degree (MSc or PhD) in medical statistics/biostatistics or an allied field (e.g. public health, epidemiology, data science) with relevant work experience Significant experience as a clinical trials, medical statistician or bio-statistician within the academic or commercial sector; a blend of both would be highly desirable A desire to apply existing and novel statistical methods to the requirements of a diverse range of statistical problems A broad understanding of cancer research Ability to lead a Clinical Trials Unit based research group As part of your online application, you will be required to upload your full CV which will pre-populate your application form, you will also be asked to attach the following documents and failure to do so will mean your application cannot be considered on this occasion: Lists of major publications, achievements, research grants and distinctions. A PDF of a maximum of five key publications, or other research outputs (e.g. patents) that best demonstrate previous productivity or a single document giving hyperlinks to these outputs. You must also complete the personal statement section of the application form in the format of a cover letter including the names and contact details of three academic referees Joining as a Group Leader, you will be given outstanding support to help you to continue to develop in your career. Along with a start-up package of funding, you will also have access to resources to establish your group, including support for recruiting key group members, such as PhD students and postdoctoral researchers. We encourage all applicants to access the job pack attached for more detailed information regarding this role. For an informal discussion regarding the role, please contact Professor Emma Hall ([email protected])
Group Leader in Cancer Stem Cell Biology
Key Requirements As part of your online application you will be required to upload your full CV which will pre-populate your application form, you will also be asked to attach the following documents and failure to do so will mean your application cannot be considered on this occasion: Lists of major publications, achievements, research grants, distinctions. Research plan (five to six pages outlining your current research interests and research programme for the next 5 years) A PDF of a maximum of five key publications, or other research outputs (e.g. patents) that best demonstrate previous productivity You must also complete the personal statement section of the application form in the format of a covering letter including the names and contact details of three academic referees Department/Directorate Information: Cancer Biology Information The Institute of Cancer Research (ICR) in London seeks to appoint a Group Leader in Cancer Stem Cell Biology to play a pivotal role in advancing our cutting-edge cancer research. The position will be based in newly-refurbished laboratory and office space at our Sutton campus within the Division of Cancer Biology. We welcome applications at both the Career Development Faculty and Career Faculty levels. The ICR is one of the world’s most influential cancer research institutions, with an outstanding track record of achievement dating back more than 100 years. In addition to being one of the UK’s leading higher education institutions for research quality and impact, the ICR is consistently ranked among the world’s most successful for industry collaboration. As a member institution of the University of London, we also provide postgraduate higher education of international distinction. One of the ICR’s key research strategies is to defeat cancer by viewing it as a dynamic ecosystem. We aim to solidify our expertise in the biology of cancer stem cells. The postholder will significantly contribute to understanding the underlying biology of cancer stem cells and how this may be exploited to address key questions in tumour relapse, disease progression and metastasis. The successful candidate will have a compelling research programme focused on cancer stem cell biology in an area which complements existing disease-specific expertise at the ICR / Royal Marsden NHS trust. Possible areas of research include (but are not restricted to) basic mechanisms of self-renewal and pluripotency, regulation of cancer stem cell fate / differentiation, how they remodel the tumour microenvironment into a supportive niche, targeting treatment resistance of cancer stem cells, and the role of CSCs in driving the metastatic cascade. Applicants must have an internationally recognised track record of leading research in cancer stem cell biology, demonstrated by high-quality publications and significant funding success. For more junior candidates, an outstanding postdoctoral track record in cancer research, coupled with a compelling research vision in a strategic area of cancer stem cell biology and clear potential to secure competitive external funding, is essential. If you would like to informally discuss this position, please contact Professor Chris Jones ([email protected]), Head of the Division of Cancer Biology at the ICR.
Employee Story
Dr Fatemeh Ahmadi Moughari is a bioinformatician working in the Functional Genomics Team, led by Rachael Natrajan, and the Bioinformatics Team, led by Syed Haider. She joined the ICR from her home country of Iran where she completed her PhD in anti-cancer drug response at Shahid Beheshti University.
"I really enjoy the multidisciplinary atmosphere here and it’s the thing that stood out when I first joined the ICR."
Industrial partnership opportunities with this group
Opportunity: A potent, orally bioavailable clinical-stage inhibitor of MPS1 with potential as a treatment for a range of cancer types including triple negative breast cancer
Commissioner: Swen Hoelder