
POETIC-A
POETIC-A is investigating whether adding a new drug alongside standard treatment reduces the chance of early breast cancer returning.
Disease site: Breast cancer
Status: Enrolling participants
Trial Documents (password protected)
What is the study about?
POETIC-A is investigating whether adding a new drug alongside standard treatment reduces the chance of early breast cancer returning.
Naturally occurring hormones can encourage the growth of breast cancer. People with oestrogen sensitive cancers receive hormone therapy to reduce the effect of oestrogen.
Aromatase inhibitors are a type of hormone therapy which is used after people have gone through menopause. These are usually given for five years after people have completed their initial breast cancer treatments (surgery, radiotherapy and sometimes chemotherapy). Although aromatase inhibitors work for most people, they can stop working as well if people have cancer that is less sensitive to hormone therapy. This means that these cancers may have a higher risk of coming back despite people taking aromatase inhibitors.
POETIC-A is investigating whether adding the drug abemaciclib alongside hormone therapy can help prevent higher risk early breast cancer from returning. Abemaciclib works by blocking molecules which encourage cancer cells to grow and divide.
POETIC-A has two parts:
- Part 1 uses samples from peoples’ breast cancer surgery to assess their cancer’s sensitivity to hormone therapy.
- Part 2 is the treatment part of the trial. People whose cancer is found to not be very sensitive to hormone therapy will be invited to join this part.
POETIC-A investigates whether giving abemaciclib in combination with aromatase inhibitors helps reduce the chance of early breast cancer with low sensitivity to hormone therapy from returning.
Who is included in the study?
POETIC-A includes post-menopausal people with early breast cancer that is oestrogen sensitive and has not spread to other areas of the body. Around 8,000 people will join part 1 of the study and 2,032 people will join part 2, from NHS hospitals in the UK.
What are the study treatments?
Part 1
Everyone who joins part 1 of POETIC-A will take a hormone therapy for at least 10 days before they have surgery to remove their cancer.
Samples of participants’ cancer removed during surgery will be tested in a POETIC-A laboratory to determine how sensitive their cancer is to hormone therapy.
Participants may also receive further treatment after surgery, such as chemotherapy or radiotherapy. This is decided by the participant with the doctors at their local hospital.
Part 2
Everyone who joins part 2 of POETIC-A will have cancer which is likely to be more resistant to hormone therapy. They will have completed any further treatments agreed with their local doctors after their surgery.
There are two treatment groups:
- Standard treatment – five years of aromatase inhibitor hormone therapy.
- Standard aromatase inhibitor hormone therapy plus abemaciclib for the first 2 years.
Participants have regular check- ups during and after their treatment, and we collect information about how they are getting on until the study is completed.
Further information for participants
Patient Information Sheet (Registration)
Patient Information Sheet (Randomisation)
A detailed summary is available on Cancer Research UK's website
POETIC-A Randomisation and Treatment Video
POETIC-A Diarrhoea Management Video
POETIC-A trial documents (password protected)
Further information for healthcare professionals
Investigator videos
The content of these videos is intended for use by healthcare professionals working on the POETIC-A study.
Rationale for the study and the role of Ki67 (Video)
What is the impact of the NICE approval for abemaciclib? (Video)
Registration to randomisation: informing and retaining patients (Video)
Managing concerns about possible side effects (Video)
Latest Study Updates
Update from the POETIC-A Chief Investigator - November 2021
Update from the POETIC-A Chief Investigator - May 2022
Update from the Trial Management Group - July 2022
The TMG and investigators discussed the impact of the recent abemaciclib license for adjuvant treatment in clinically high-risk patients.
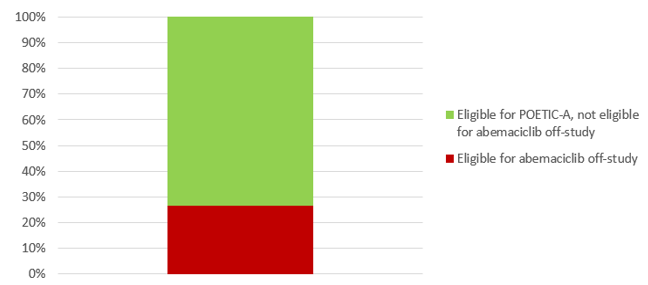
Data from the POETIC trial (Figure below) showed that only 27% of patients who would be eligible for randomisation in POETIC-A will meet the clinical criteria to access abemaciclib under the NICE guidance. For the remaining 73% of patients with high biological risk (due to elevated KI67), POETIC-A will be an important opportunity to potentially derive benefit from abemaciclib.
Investigators noted that as abemaciclib becomes more embedded in clinical practice, this may help recruitment to POETIC-A.
 Contact details and regulatory information
Contact details and regulatory information
Chief Investigator: Professor Stephen Johnston, The Royal Marsden NHS Foundation Trust
Coordinating Investigator: Dr Alistair Ring, The Royal Marsden NHS Foundation Trust
ICR-CTSU scientific/methodology lead: Professor Judith Bliss
Surgical lead: Professor Cliona Kirwan, University of Manchester and Manchester University NHS Foundation Trust
Translational study lead: Professor Mitchell Dowsett, The Ralph Lauren Centre for Breast Cancer Research, The Royal Marsden NHS Foundation Trust
Bioinformatics and genomics analysis lead: Dr Maggie Cheang, The Institute of Cancer Research
Trial management contact: [email protected]
Sponsor: The Institute of Cancer Research
Funding: Lilly, Cancer Research UK (via ICR-CTSU Programme)
Trial identifiers
EUDRACT number: 2019-003897-24
REC reference: 20/LO/2019
CPMS ID: 44805
ClinicalTrials.gov: NCT04584853
Publications and presentations
There have been no presentations or publications to date.

