Image: An illustration of how the R2KD tool works
Credit: Dr Maggie Liu, first author
Researchers have developed an investigative procedure that makes the early stages of drug discovery more efficient. This test can help scientists identify new biologically active compounds that can be used to develop effective medications.
Scientists worldwide can use the new procedure to select only the compounds with the most potential, preventing them from wasting time and resources on those that would ultimately prove ineffective.
This work, carried out by researchers at the Centre for Cancer Drug Discovery at The Institute of Cancer Research, London, is ground-breaking in the field of fragment-based drug discovery, which has become the standard way to identify the starting point for a drug discovery programme.
The study was funded by Cancer Research UK, and it has been published in the Journal of Medicinal Chemistry.
Turning the approach from qualitative to quantitative
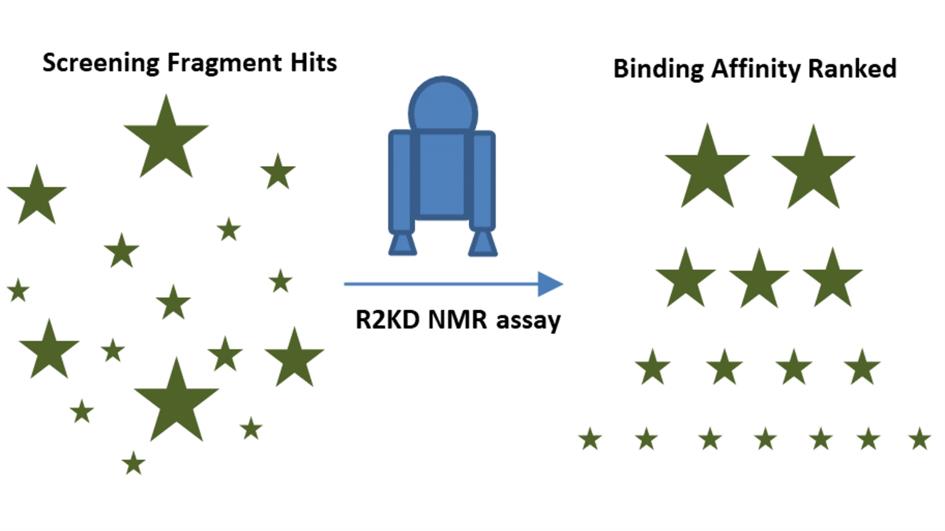
Fragment-based drug discovery uses various techniques to screen libraries of compounds, or fragments, with a low molecular weight. During the initial stage, sometimes referred to as the hit-finding phase, scientists introduce a potential anti-cancer target and see which of the fragments interact with it.
Previously, it was only possible to qualify the interactions between fragments and target proteins, meaning that researchers could determine that ‘yes, an interaction occurred’ or ‘no, there was no interaction.’ It was difficult to apply this to very weak interactions, which were not a clear yes or no, and the team could not rule out nonspecific interactions.
Now, thanks to this latest research, researchers can quantify these interactions by determining their strength. The ability to rank the fragments in order of interaction strength allows research teams to identify those that are truly active and worth following up. This extra information can give them the confidence to take a fragment forward to the next stage of research, during which they will optimise it by refining its shape, joining it with other fragments, or both.
First author Dr Maggie Liu, Senior Scientific Officer in the Cancer Therapeutics – Medicinal Chemistry 4 Team at the ICR, said:
“We are confident that this procedure will become part of the standard drug discovery workflow. Any teams with the right equipment can use our method, so we hope it will benefit the wider drug discovery community. At the ICR, we have already started follow-up work on one fragment that we identified using the new test, and we plan to use this tool on all future projects.”
Testing the R2KD tool
Dr Liu and her team used a drug discovery approach called ligand-observed nuclear magnetic resonance (LONMR), which is similar in principle to how an MRI scanner works. They chose to use a known complex as the target, which earlier work had already cross-validated.
While screening a library of fragments, they observed the transverse relaxation rate (R2) of the fragments, which indicates the speed at which they tumble in the solution. As large molecules tumble more slowly than small ones, an increase in a fragment’s R2 value was a sign that it had interacted with a target protein.
The researchers then applied a new mathematical formulation to determine a binding affinity value (Kd) for each fragment. The Kd values were compared to reveal the fragments that had the strongest interactions. Based on these steps, the team named their test ‘R2KD.’
Dr Liu said:
“We know that our tool works in a wide Kd range of binding events, extending from 10 µM to 1 mM. This is the most promising range and, therefore, the most practical choice. Very strong interactions that would fall outside of this range are unlikely when using fragments, and weaker interactions would make it difficult to develop the fragment into a drug.”
Scientists working in drug discovery still face challenges. At the moment, it is only possible to screen 1,000–2,000 fragments in one go, which limits the use of a library. Exploring a wider range of fragments at a time would make the process more efficient. The sensitivity of the NMR system is also a limiting factor, and false positives continue to be a significant issue.
However, by allowing researchers to get quantitative results from their screenings, the R2KD test removes a major obstacle.
A cumulative research process
The development of the tool was a long piece of work that involved multiple projects, bringing together different teams from across the ICR. Dr Liu’s team drew on the expertise of colleagues working in various areas within the institute.
Dr Liu said:
“It was a very long piece of work – the original idea was conceived about five years ago! We learnt from each project, applying the findings to the next one so that we could learn more. We are thrilled to have developed a new tool that will make it easier and quicker to find the drugs of the future. It’s exciting to think that the latest fragment we have identified might go on to form a drug that saves thousands of lives.”
The researchers are also hopeful that other scientists will be able to use the tool to identify new biologically active compounds of their own.
Professor Swen Hoelder, Head of Chemistry in the Centre for Cancer Drug Discovery at the ICR, said:
“Fragment-based drug discovery is a powerful approach for identifying chemical starting points for drug discovery programs. Our work has made a significant contribution to refining the process and maximising its efficiency. We are using the R2KD method for several of our drug discovery programs, and we believe it will make NMR-based fragment drug discovery more accessible to research teams in academia and industry.”
In our Centre for Cancer Drug Discovery, we are working to create more and better drugs for people with cancer. But the life-saving research that is taking place within it relies on your support. Please donate today to help more people survive cancer.