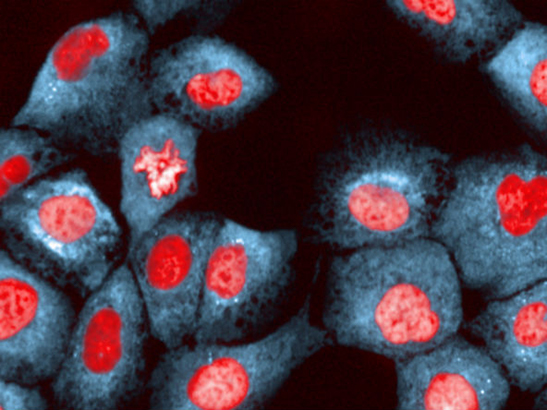
In the study, ER-positive breast cancers were found to evade anti-hormone treatments by mimicking oestrogen. This image shows breast epithelial cells under ER stress (photo: Chris Bakal)
Common breast cancers could be using a cholesterol-based molecule to evade standard anti-hormone treatments, causing patients’ disease to return, according to new research.
In a new study in the journal Breast Cancer Research, scientists at the Breast Cancer Now Toby Robins Research Centre at The Institute of Cancer Research, London, have identified that oestrogen receptor (ER)-positive breast cancers produce a molecule made from cholesterol, called 25-hydroxycholesterol (25-HC). This can mimic oestrogen and encourage cancer cells to continue growing without it, as patients undergo anti-hormone treatment.
Up to 40,000 women are diagnosed with ER-positive breast cancer each year (accounting for up to 80% of all breast cancers), meaning that they have more ER than normal breast cells and are particularly sensitive to oestrogen, relying heavily on it to grow and spread.
Anti-hormone drugs are used to these patients, working by either reducing oestrogen in the body (known as aromatase inhibitors), by blocking the receptors themselves (tamoxifen), or by destroying the receptors entirely (fulvestrant).
'Hugely significant'
While these treatments have significantly improved survival rates in recent decades, around 12,000 ER-positive patients currently suffer a recurrence during or following endocrine treatment.
Dr Lesley-Ann Martin, Group Leader at the Breast Cancer Now Toby Robins Research Centre at the ICR, said: “During the course of treatment, ER-positive breast cancers that are ‘fed’ by oestrogen often become resistant to standard hormone therapy. Our research has demonstrated that these cancer cells can use a cholesterol molecule to mimic oestrogen so that they continue to grow without it.
“This is hugely significant. Testing the patient’s tumour for 25-HC or the enzymes that make it may allow us to predict which patients are likely to develop resistance hormone therapy, and tailor their treatment accordingly.
“Our study also demonstrates that statins could be a valuable addition to breast cancer treatment, and that this warrants investigation in clinical trials.”
The study was funded predominantly by Breast Cancer Now — made possible by The Eranda Rothschild Foundation — and was part-funded by the National Institute for Health Research Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and the ICR.
Bypassing the lack of oestrogen
Cholesterol is an important molecule that allows the body to build and maintain cell membranes and produce a number of hormones. As well as obtaining cholesterol from food, the body produces its own cholesterol in a process called the cholesterol biosynthesis pathway.
Using breast cancer cells grown in the lab, a team of scientists led by Dr Martin investigated the processes that cause women with ER-positive breast cancer to relapse while taking aromatase inhibitors (AIs), generally taken for five years after surgery.
The researchers grew ER-positive cells in the absence of oestrogen, mimicking the cells found in tumours being treated with AIs. They found that the cholesterol biosynthesis pathway was enabling the cells to make their own fuel by producing 25-HC, allowing them to continue growing despite a lack of oestrogen.
Using cell-line models, the team found that blocking the production of parts of this cholesterol-production mechanism slowed down the proliferation of the cancer cells by 30-50%.
The lab-based findings were corroborated using clinical data from ER-positive patients. In 124 patients from two independent studies, the researchers found that the overactivation of certain genes involved in cholesterol biosynthesis (EBP, LBR, MSMO1 and SQLE) were significantly associated with poorer response to anti-hormone treatments.
Statin trials 'needed'
Of particular note, in a separate cohort of 747 patients, increased expression of the SQLE gene was found to be significantly associated with a higher chance of recurrence up to 10 years later.
While early research, the potential implications of this finding on the treatment of ER-positive breast cancer could be two-fold, if confirmed.
The finding could one day enable scientists to test whether a patient’s tumour might become resistant to AIs by seeing if it is over-producing cholesterol, using 25-HC or other proteins involved in the cholesterol biosynthesis pathway as a biomarker in a tumour test. If likely to develop resistance, these patients could be switched to different drugs that destroy the oestrogen receptors entirely, such as fulvestrant.
The study adds further weight to the argument that a clinical trial to test whether cholesterol-lowering statins improve the effectiveness of anti-hormone therapies for patients with ER-positive breast cancer is needed.
'Crucial discovery'
While previous epidemiological evidence does not support an association between statin use and reduced breast cancer incidence, it does support the idea that statins could improve prognosis for people who already have breast cancer.
Baroness Delyth Morgan, Chief Executive at Breast Cancer Now, said: “This is a really crucial discovery. Far too many women have to deal with the potentially devastating consequences of their breast cancer coming back and this research presents an important opportunity to improve the effectiveness of today’s most commonly used treatments.
“This study breaks new ground in uncovering how some breast cancers continue to survive without oestrogen and suggests that women could benefit from adding statins to standard anti-hormone treatments.
“But this is early research and greater clinical evidence is now needed to understand the potential risks and benefits of this approach.”