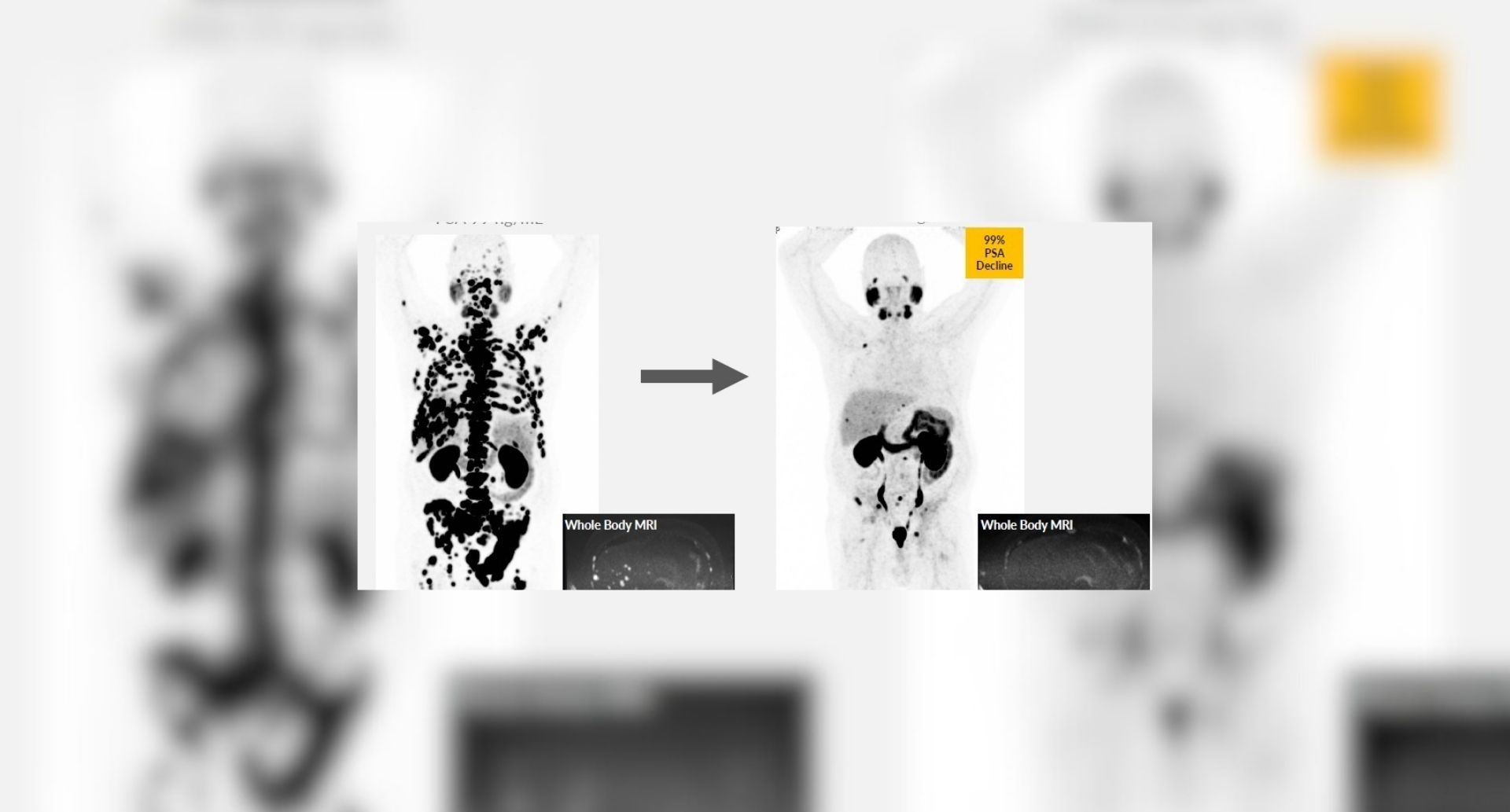
Practice-changing clinical trials in radiotherapy and imaging
We led major clinical trials in radiotherapy and imaging which have changed standard clinical practice for cancer treatment.
We have led major clinical trials in radiotherapy and imaging which have changed standard clinical practice for cancer treatment, forming the basis of NICE and international guidelines and helping set standard care in the UK.
The START trials introduced a new schedule for treatment of breast cancer with radiotherapy.
There had been a historical assumption that a high overall dose of radiotherapy delivered in many small doses (fractions) over time would deliver the greatest anti-tumour effect with the least damage to healthy tissues. However, preliminary evidence from the mid-1980s suggested that breast cancer might be an exception.
Breakthrough trials
To test for benefits and side-effects of a lower total dose in fewer, larger fractions, Professor Judith Bliss and Professor John Yarnold designed and led three randomised clinical trials in women with early-stage breast cancer: a pilot in 1986 followed by the two START trials in 1998.
The START trials, led by The Institute of Cancer Research, London and The Royal Marsden NHS Foundation Trust, compared the international standard regimen of 25 fractions over five weeks with novel schedules giving fewer, larger fractions to a lower total dose.
They found that comparable levels of cancer control were achieved when treatment was given in a lower overall dose in fewer, larger fractions. In addition, there was a 20% reduction in chronic side-effects for the 15-fraction schedule delivered in three weeks instead of the standard 25-fraction regimen delivered over five weeks.
Far-reaching impact
The study has had widespread impact on UK and international clinical practice. NICE introduced guidance in 2009 recommending replacing the 25-fraction schedule with 15 fractions in 3 weeks and this has been adopted across the UK, benefiting around 25,000 women a year with early breast cancer.
Another example is the MARIBS trial, which changed practice in breast cancer screening for women likely to be carrying a BRCA mutation.
Professor Martin Leach and colleagues at the ICR recognised the potential value of detecting cancer early in women who were probable BRCA mutation carriers, as cancer can occur at a younger age in this group, with the disease often progressing more rapidly.
A more sensitive approach
They designed a national multicentre clinical study, MARIBS, in women aged 35-49 years with a strong family history of breast cancer. This tested the effectiveness of screening for tumours using magnetic resonance imaging (MRI) compared with X-ray mammography in women who were probable BRCA mutation carriers and at increased risk of breast cancer.
Over the study period, the participants had both X-ray mammography and MRI screening annually, and the number of cancers detected by each method was counted. The ICR researchers found that screening in these women using MRI was a significantly more sensitive approach, and this was particularly pronounced in BRCA mutation carriers.
Results from this trial formed the foundation of new NICE and international guidelines on MRI screening, with the technique becoming standard care in the UK for women likely to have BRCA mutations.
Patients all over the world are benefiting from these changes in clinical practice as a result of ICR research.
Related news
Related blogs
Professor Paul Workman was 37 and already well established as a cancer researcher when his mother, Ena, died aged 68 from a rare bone tumour known as chordoma. About one in a million people are affected by the condition, for which there are no targeted drug treatments.