Identification of IRE1 inhibitors using a 384-well DELFIA assay
Inositol-requiring enzyme 1 alpha (IRE1α) is a transmembrane sensor protein with both kinase and ribonuclease activity which plays a crucial role in the unfolded protein response (UPR).
Protein misfolding in the endoplasmic reticulum (ER) lumen triggers dimerisation and subsequent trans-autophosphorylation of IRE1α. This leads to the activation of its endoribonuclease (RNase) domain and splicing of the mRNA of the transcriptional activator XBP1, ultimately generating an active XBP1 (XBP1s) implicated in multiple myeloma survival.
In collaboration with Professor Ian Collins and Dr Faith Davies and led by Dr Rosemary Burke, we have developed a 384-well screening assay using DELFIA® technology that is specific for IRE1α autophosphorylation. Using this format a focused library of 2312 potential kinase inhibitors was screened and several novel IRE1α kinase inhibitor scaffolds were identified (see Fig 1 below).
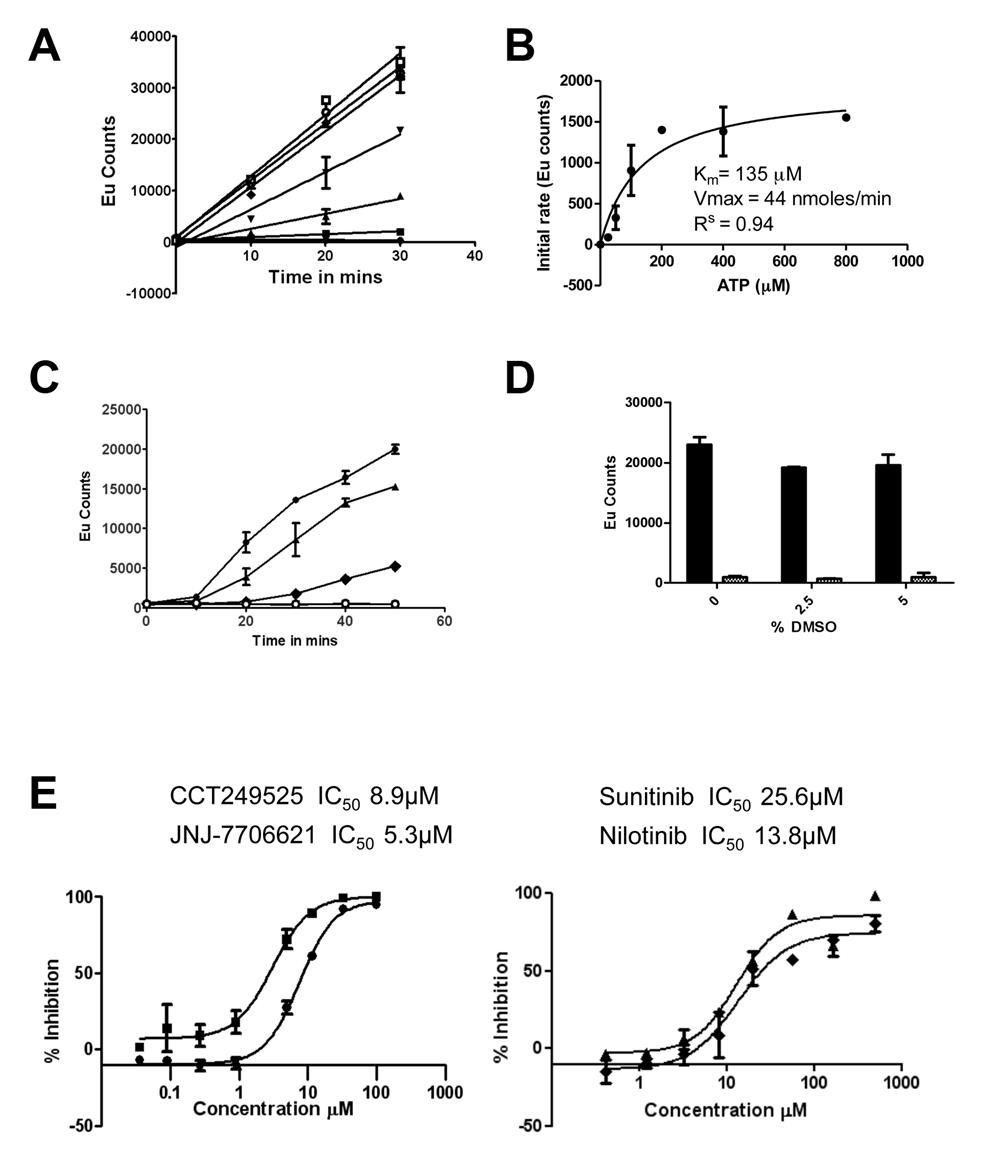
Figure 1. The IRE1α autophosphorylation DELFIA® assay. (A) Time course with varying ATP concentrations used to calculate the Km for adenosine triphosphate (ATP) (● no ATP, ■ 25 µM ATP, ▲ 50 µM ATP, ▼100 µM ATP,♦ 200 µM ATP, ○ 400 µM ATP,□ 800 µM ATP). B) Determination of the Km for adenosine triphosphate (ATP). C) Enzyme reaction time course; mean +/- SD of n=2 wells (● enzyme reaction at 700 nM IRE1α and 100 µM ATP, ▲ enzyme reaction at 500 nM IRE1α and 100 µM ATP, ♦ enzyme reaction at 350 nM IRE1α and 100 µM ATP,○). Enzyme reaction at 700 nM IRE1α without ATP). D) Effect of DMSO on the IRE1α autophosphorylation DELFIA®, showing no effect on the assay with up to 5% DMSO. E) Inhibition of IRE1α autophosphorylation activity by CCT249525 (●) , JNJ-7706612 (■), Sunitinib (♦) and Nilotinib (▲).