Search Issue 52 | Summer special edition 2025
Editorial
Cancer drug discovery has arguably never been more exciting. Our knowledge base is rapidly expanding, and our research techniques are becoming increasingly sophisticated, complex and innovative.
Such rapid developments have led to a brighter future for many people with cancer, who have a better chance than ever before of surviving the disease. Now, we need to focus our efforts on the continuing areas of unmet need, so we can provide hope to more patients and their families.
To this end, we are taking every opportunity to acquire the brightest talent, promote collaboration between teams and optimise our experimental techniques so that we can boost our preclinical work. We want to ensure that only drugs with true potential move into clinical trials, and we want to maximise the number of these coming through the pipeline.
We have created a special edition of Search to highlight our quest to discover and develop new cancer treatments. Here, we take you step by step through the creation of a drug – from understanding the biology of cancer to designing new prototype drugs to testing them in clinical trials. Along the way, you will learn about some of our specific projects and meet several of our researchers.
We’re home to one of the most successful academic cancer drug discovery and development centres in the world, and we want to keep pushing the boundaries of what’s possible. With our expertise and your generous support, I’m confident that we’re on the way to discovering new drugs that will transform the lives of cancer patients worldwide.

Thomas Bland
Deputy Director of Development
The Institute of Cancer Research
Cancer biology
Understanding the biology of cancer
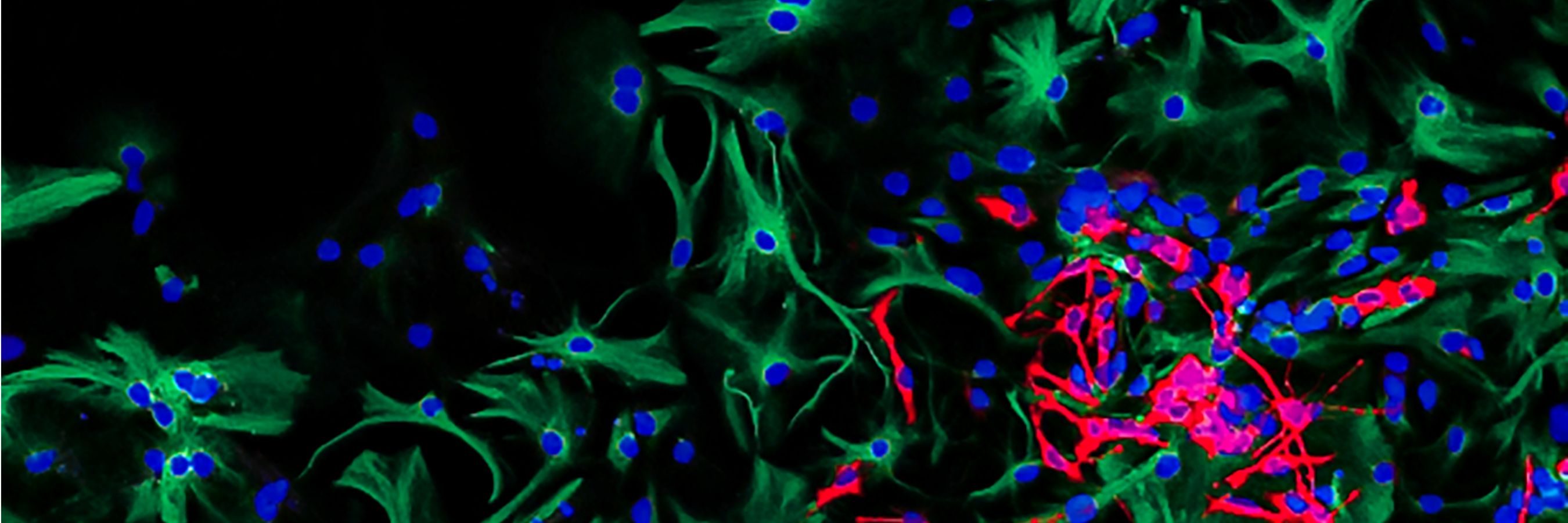
To develop new targeted treatments, our researchers need to uncover the cellular and molecular changes underpinning different cancers.
Cancer still has many secrets we are working to uncover:
- Cancer cells can change over time as genetic mutations alter their appearance or behaviour. Some changes help the cells survive, and they can pass this advantage on when they divide. This can sometimes lead to treatment resistance, where a therapy stops working. Our researchers are working on exploiting this by altering cancer’s environment to make the cells evolve in a way that renders them more susceptible to treatment.
- Complex interactions between the immune system and cancer can influence the mutations promoting the disease’s growth and spread. Our scientists, including Dr Annie Baker, have developed a technique for analysing how the immune system responds to cancer cells with different mutations as the disease progresses.
- The microbiome – the collection of microbes in and on the body – has links to cancer evolution. After finding that a specific variation of a common bacteria seems to increase the risk of colon cancer, our researchers are working to better understand the role of gut bacteria in cancer initiation and progression.
- Non-cancerous cells close to tumours can affect how a treatment responds. Our scientists are researching the many different proteins that these cells secrete, some of which can increase cancer’s resistance to chemotherapy.
By understanding all these factors, our scientists will have a better idea of how cancer develops, spreads and becomes resistant to treatment, which will help them find new ways to prevent and treat the disease.
“The devastating impact of losing my father to brain cancer when I was young motivated me to study science and try to make a difference to cancer patients and their families.
“Our team is hoping to design treatments that alter the trajectory of cancer evolution in a way that allows the immune system to recognise and kill cancer cells.
“Understanding the fundamental biology driving cancer will help us develop better treatments, drug combinations and dosing schedules. Ultimately, we will ensure that cancer patients have kinder treatments and live for longer.”
Dr Annie Baker, Senior Staff Scientist, Genomics and Evolutionary Dynamics Group

Small molecule drugs are helping revolutionise cancer treatment. These chemically synthesised compounds have a low molecular weight that allows them to enter cells easily, so they can target cancer at its foundations by tackling the proteins that drive it.
Designing new cancer drugs
Using small molecule drugs to destroy cancer proteins
Proteins are one of the key molecules in our body, and they are essential for every cell function, including growth, signalling and survival. When they become faulty or overactive, they can drive cancer. Traditionally, cancer therapies have aimed to block the activity of these proteins, but this often proves challenging.
Targeted protein degradation is a cutting-edge approach that kills cancer cells – not by temporarily inhibiting cancer-driving proteins but by removing them entirely. It uses specially designed small molecules such as PROTACs and molecular glue degraders, which cause the cancer target protein to interact with a cellular enzyme called E3 ligase. E3 ligase uses a marker called ubiquitin to flag the protein for degradation by the cell’s natural recycling system.

“Targeted protein degradation is revolutionising cancer drug discovery, offering new hope for patients. With novel small molecular degraders, we’re opening up new possibilities for treating cancers that have been resistant to traditional therapies.”
Dr Agnieszka Konopacka, Group Leader, Induced Proximity Therapeutics Group
Unlike inhibitor drugs, which stop a protein from working, degradation-based therapeutics offer the potential to target cancer proteins previously considered undruggable. Effective in lower doses, they reduce the risks of side effects and drug resistance – two major hurdles in cancer therapy.
Dr Agnieszka Konopacka leads drug discovery biology at our Centre for Protein Degradation. Her research team develops models and tools to study molecular glue degraders and PROTACs at a highly detailed level. The team is also working to discover new cancer targets and E3 ligases to help patients with hard-to-treat cancers.
With our strong legacy in drug discovery and focus on developing innovative technologies, we are shaping a future where challenging cancers can be treated effectively and safely.
Structural biology in drug design
The detail behind how drugs are designed
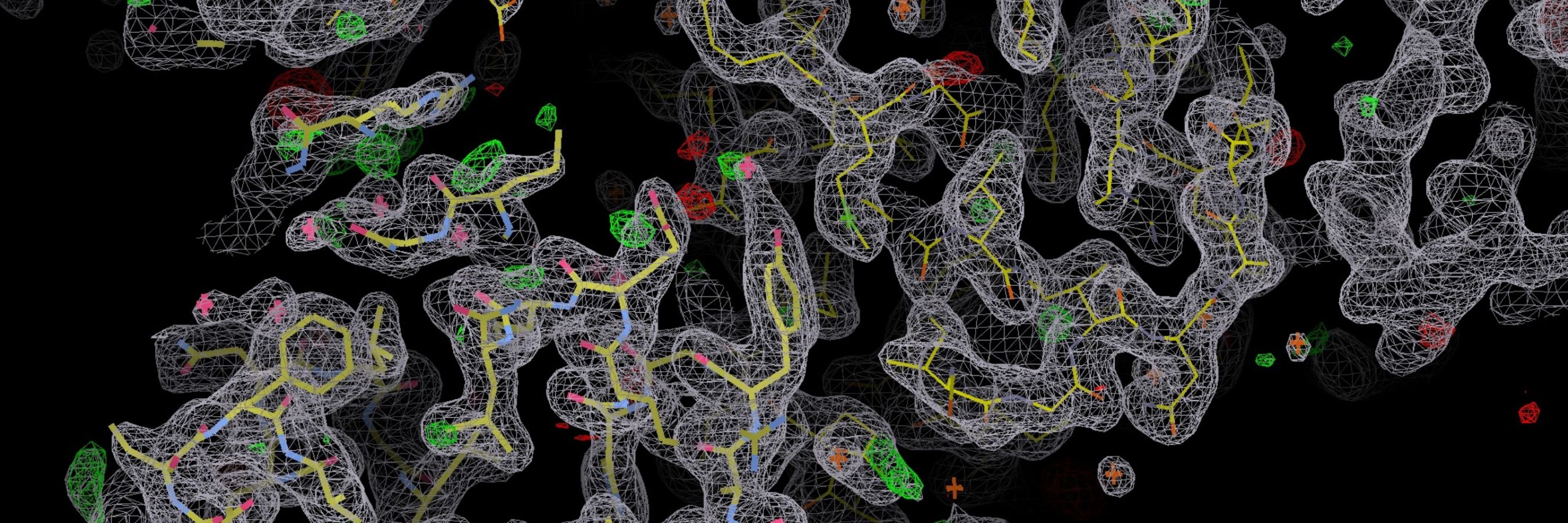
To design drugs that perfectly target a particular protein on a cancerous cell, scientists need to fully understand the atomic structure of the protein.
Historically, we have achieved this using a technique called X-ray crystallography, which involves passing an X-ray beam through a specially prepared sample (protein crystal) and then using the diffraction pattern to reconstruct the molecule’s three-dimensional structure.
However, this approach is not suitable for all biological molecules, particularly those that are larger or more complex. As a result, cryo-electron microscopy (cryo-EM) has become popular. This cutting-edge technique reveals the detailed structure of a biological molecule by rapidly freezing samples to below -150°C before passing a beam of electrons through them to collect images from different angles. A computer can use these to create a three-dimensional model of the molecule.
Our scientists, including Victoria Cushing, recently led the development of an improved cryo-EM process that produces higher-resolution images in a significantly shorter timeframe. The first stage involves rapid screening using a more accessible cryo-EM instrument, during which the researchers assess the resulting images to select the specimens with the highest potential. In the second stage, a high-end microscope – which has a cold field emission gun machine that emits a highly coherent electron beam – is used to provide more detailed images of the selected molecules.
Using this approach, researchers can achieve exceptional images showing individual atoms and their interactions. They can also work faster, so they can determine the structure of multiple protein complexes each day.
This methodological advance has overcome a longstanding technical challenge, increasing both resolution and speed. By providing researchers with such a detailed view of molecules of interest, it has the potential to aid drug discovery on a large scale – across research institutions worldwide and across cancer types.
“My work is crucial for understanding how proteins fold, how they interact with other molecules and how drugs might bind to them.
“This detail-orientated work is fascinating, and it’s exciting to think that some of the protein structures and interactions we uncover could be the key to new, effective treatments that go on to save thousands of lives.”
Victoria Cushing, PhD student in Dr Basil Greber’s research team, Structural Biology of DNA Repair Complexes Group
Improving the success rate of new cancer drugs

“At the ICR, I’m able to interact with an interdisciplinary team of amazing biologists, chemists, pharmacologists, medics and computer scientists, all of whom are working together to achieve the same goal but addressing the problem from different angles.”
Dr Joanna Loizou, Deputy Director, Centre for Target Validation
The creation of new personalised cancer medications begins with identifying and validating new drug targets. Scientists must find a protein in the body that has a key role in cancer and then demonstrate that another substance can interact with it to achieve a therapeutic effect.
Once they have discovered a potential cancer target, they face the significant challenge of getting it into a drug discovery programme to progress it to clinical trials. To bridge this gap, we established the Centre for Target Validation (CTV), which sits within the Centre for Cancer Drug Discovery.
The CTV aims to identify candidate targets from across the wide spectrum of our laboratories and carry out in-depth therapeutic target validation to inform the launch of successful drug discovery projects.
The team benefits from the leadership of Dr Joanna Loizou, a specialist in genome stability and DNA repair pathways with a strong background in translational medicine. Dr Loizou, who joined us in November 2024 to take on the role of Deputy Director of the CTV, said:
“Our centre was created to select and validate targets identified by the world-class research at the ICR. Using a fast and streamlined approach, we can take targets through to drug discovery projects, which we have the necessary facilities and expertise to lead onsite.
“Failure rates of drugs in clinical trials are high, and a principal reason of failure is lack of effectiveness. By having a robust target identification and validation dataset and using the right preclinical experiments, we should be able to improve the success rate of new cancer drugs, which we hope will be more effective and better tolerated.”
The CTV will incorporate the newest available techniques into its workstreams and draw on both publicly available data and its own broad target validation datasets to select targets with the most potential.
“We’re looking for targets with clinical rationale,” said Dr Loizou. “For instance, those with molecules that might be sensitive to the inhibition of another protein. If the chemistry is right, we should be able to get all the way to the clinic.”
Cancer discovery news
Our researchers are making the discoveries that defeat cancer. Read the latest findings from our world-leading research.