Lobular Carcinoma of the Breast
10 to 15% of breast cancers are of a special pathological type called lobular carcinoma. Lobular carcinomas are typically slow growing, very sensitive to hormones and have a high risk of late recurrence and a unique metastatic pattern. They tend not to respond well to standard therapies. Progress in our understanding of this particular type of breast cancer has been hampered by the lack of models to study it.
We have recently shown that lobular breast cancer cells either from cell lines or from patient tumors when grafted directly to the milk ducts of immunocompromised female mice, grow, invade, and metastasize in a similar way as they do in patients.
Molecular analysis of purified lobular carcinoma cells from intraductal xenografts revealed that these tumor cells actively secrete and modulate extracellular matrix.
Blocking the secreted enzyme, LOXL1 that is critical for this modulation interferes with tumor growth and progression (Sflomos G et al, EMBO Mol Med. 2021).
We will test whether this tumor cell-derived matrix provides a vulnerability that can be exploited therapeutically.
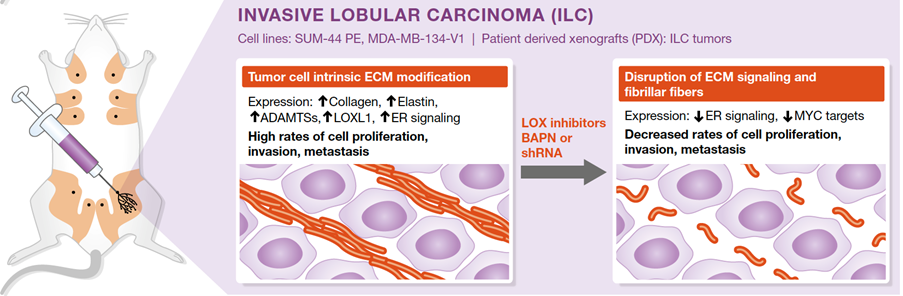
Image: The intraductal (MIND) system reveals lobular carcinoma-specific biology and vulnerabilities. Adapted from Kozma et al.
New Patient-Derived In Vivo Models
In close collaboration with clinical partners we aim to establish a panel of patient-derived MIND models that reflects the clinical complexity of the disease both in terms of tumour subtypes and patient genetic ancestry.
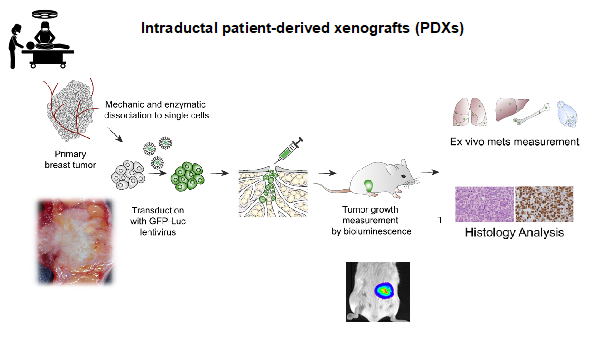
Image: Workflow of the MIND patient-derived model establishment. Courtesy Valentina Scabia.
Resources
References
Sflomos, G., Battista, L., Aouad, P., DeMartino, F., Scabia, V., Ayyanan, A., Ifticene-Treboux, A, RLS, Bucher, P., Ambrosini, G., Fiche, M.,, Brisken, C. Intraductal Xenografts Model Lobular Carcinoma of the Breast and Reveal Matrix Remodeling by LOXL1 as a Targetable Hallmark
EMBO Mol Med. 2021.
Previewed: Kozma KJ, Done SJ, Egan SE. The tumor cell-derived matrix of lobular breast cancer: a new vulnerability. EMBO Mol Med. (2021)
Koch, C, .Kuske, A., Josse, S.A., Yigit, G., Sflomos, G., Thaler, S., Smit, D.J., Werner, S., Borgmann, K., Gärtner, S., Mohammadi, P.M., Battista, L., Cayrefourcq ,L., Altmüller, J., Salinas-Riester, G., Raithatha, K., Zibat, A., Goy, Y., Ott, L., Bartkowiak, K., Tan, Z. T., Speicher, M.R., Müller, V., Jücker, M., Thiery, J.P., Brisken, C.*, Riethdorf, S.*, Alix-Panabières, C*., Pantel, K. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity *equal contribution
EMBO Mol Med. (2021) Sep 7;12(9):e11908.
Cartaxo, AL., Estrada, M., Domenici, G., Roque, R., Silva, F., Gualda, EJ., Loza-Alvarez, P., Sflomos, G., and Brisken, C. , Alves, PM., André, S., Brito, C. A novel culture method that sustains ERα signaling in human breast cancer tissue microstructures
J Exp Clin Cancer Res 2020 Aug 17;39(1):161.
Siersbæk, R., Scabia, V., Chernukhin, I., Papachristou, E., Broome, R., Green, A., Joosten, S., Kumar, S., Alvarez, R., Nagarajan, S., Glont, S., Omarjee S., Aitken, S., Kishore,K., Rakha, E., D’Santos, C., Zwart, W., Russell, A., Brisken, C, Carroll, J. IL6/STAT3 co-opts ER regulatory elements to drive metastasis in breast cancer.
Cancer Cell. 2020 Jul 6:S1535-6108(20)30311-1.
Laszlo, C. F., De Martino, F., Montoya, J. P., Shamseddin, M., Beguin, A., Nellen, R., Bruce, S., Moniatte, M., Henry, H., and Brisken, C. A high resolution LC-MS targeted method for the concomitant analysis of 11 contraceptive progestins and 4 steroids
J Pharm Biomed Anal. 2019 Oct 25;175:112756.
Vincent, GP., Nacht, AS, Ferrari, R., Zaurin, R., Scabia, V., Carbonell, J., Le Dily, F., Quilez, J., Leopoldi, A., Brisken, C, Beato, M. C/EBPα mediates the growth inhibitory effect of progestins on breast cancer cells
EMBO J. 2019 Sep 16;38(18):e101426.
Fiche, M., Scabia, V., Aouad, P., Battista, L., Treboux, A., Stravodimou, A., Zaman, K., RLS, Ayyanan, A., Sflomos, G., and Brisken, C. Intraductal patient derived xenografts of ER+ breast cancer recapitulate the histopathological spectrum and metastatic potential of human lesions
J Pathol. 2019 Mar;247(3):287-292.
Sflomos, G., Dormoy, V., T. Metsalu, T., Jeitziner, R., Battista, L., Scabia, V., Raffoul, W., Delaloye, J.-F., Treboux, A., Vilo,J., Fiche, M., Ayyanan, A., Brisken C. (2016) A robust preclinical model for ERα positive breast cancer points to the mammary epithelial microenvironment as critical determinant of luminal phenotype and hormone response
Cancer Cell, 2016 Mar 14; 29(3):407-422.
Previewed: Haricharan S, Lei J, Ellis M. Mammary Ductal Environment Is Necessary for Faithful Maintenance of Estrogen Signaling in ER(+) Breast Cancer.
Cancer Cell. 2016 14;29(3):249-50.
Featured in: Research Watch: Bledsoe, K. Milk Duct Engraftment Allows Preclinical Modeling of ER+ Breast Cancer Milk Duct Engraftment Allows Preclinical Modeling of ER+ Breast Cancer
Cancer Discovery. 2016