Two decades ago, a pivotal exchange between two scientists sparked a scientific and medical revolution. Professor Alan Ashworth, then Director of the Breast Cancer Now Toby Robins Research Centre at The Institute of Cancer Research, London, and his collaborator Professor Steve Jackson had long known that inherited genetic mutations in the genes BRCA1 and BRCA2 increase the risk of cancer. But, they wondered, what if those same mutations could be turned against the cancer itself?
This idea sparked the development of PARP inhibitors – drugs now used to treat tens of thousands of people with BRCA-related cancers, including breast, ovarian, pancreatic and, more recently, prostate cancer. It was a leap of logic based on a genetic principle called synthetic lethality, which was translated to clinical use through rigorous laboratory research, industry collaboration and a shared vision of smarter, more targeted therapies.
To mark 20 years since that moment of insight, Robbie Lockyer spoke with Professor Chris Lord, Deputy Head of the Breast Cancer Research Division and the Deputy Director of the Breast Cancer Now Toby Robins Research Centre at The Institute of Cancer Research (ICR). Professor Lord was part of the team that helped take the concept of synthetic lethality from bench to bedside.
What is synthetic lethality?
Synthetic lethality is a genetic concept where the loss of function in either one of two genes individually has little to no effect on cell survival – but losing both kills cells. This can be exploited in cancer treatment, as cancer cells often already carry mutations in one of these genes. If a cancer already has a mutation in one gene, such as BRCA1 or BRCA2, targeting its synthetic lethal partner gene causes the cancer cell to die but leaves normal cells unharmed.
One of the most well-known synthetic lethal targets is PARP – a protein involved in repairing damaged DNA. BRCA genes and PARP operate in complementary repair pathways. So, if a cancer cell already has a faulty BRCA1 or BRCA2 gene, inhibiting the PARP protein – which normally helps repair DNA – overwhelms the cell’s ability to fix that damage – leading to cancer cell death.
Professor Lord said: “This is the basis for PARP inhibitor therapies used in BRCA-mutated cancers. Cancer cells with BRCA mutations are already limping along. Take out PARP and they can’t cope. But normal cells, which still have BRCA working, can survive.
“It’s a subtle yet powerful idea that flips the usual approach to cancer treatment. Instead of targeting something the cancer has too much of, it targets what the cancer lacks.”
Looking back: discovering a new way to kill cancer cells
Professor Lord joined the ICR in 2000, not long after Professor Mike Stratton and Professor Ashworth mapped, understood and cloned BRCA2 in breast cancer.
Shortly after, Professor Andrew Tutt, now Head of the Division of Breast Cancer Research and Director of the Breast Cancer Now Toby Robins Research Centre at the ICR, who was working in Professor Ashworth’s lab at the time, showed that BRCA-deficient cells could not repair certain kinds of DNA damage. Around the same time, other researchers, including those outside the ICR, demonstrated that these cells were particularly sensitive to platinum chemotherapy, causing DNA damage that requires BRCA-mediated repair. This helped build the case for targeting DNA repair vulnerabilities in BRCA-mutant cancers.
Two of Professor Lord’s former postdoc colleagues, Dr Nuala McCabe and Dr Hannah Farmer, made a critical observation. They found that a class of existing PARP inhibitors could kill BRCA-deficient cells directly, without the need for chemotherapy or radiotherapy.
Professor Lord said: “That was the spark that ignited everything. This wasn’t just lab trivia. The cells were dying with one drug alone – a potential single-agent treatment for cancers that previously had very few effective options. We could see this was different, and I remember there being real excitement in the lab.”
Translating discovery
This excitement was quickly channelled into action through an early collaboration with KuDOS, a biotech company founded by University of Cambridge scientist Professor Jackson in 1997. In the early 2000s, a pivotal exchange between Professor Jackson and Professor Ashworth helped bring together two complementary ideas – BRCA-related DNA repair defects and the potential of PARP inhibition. KuDOS had already developed a potent PARP inhibitor called olaparib.
Although the concept of synthetic lethality has broad potential applications, its earliest clinical translation centred on BRCA-mutant cancers – particularly ovarian and breast cancer – where DNA repair defects were already well understood. While prior approaches to using PARP inhibitors in cancer had focussed on using these to enhance existing cancer treatments, it was the ICR’s discovery that these drugs could be used as targeted drugs on their own in BRCA-deficient tumours that ultimately shaped the direction of their clinical development.
Professor Lord said: “Back then, at the start of the millennium, academic-industry collaborations weren’t as common as they are now. However, KuDOS had done a phenomenal job developing these molecules. Without their work, we wouldn’t have had the tools we needed.”
Elsewhere, a team working with Professor Thomas Helleday at Sheffield University made a similar observation about BRCA-deficient cells and PARP inhibitors.
Professor Lord said: “We soon became aware that we were all working on something similar. Instead of competing, we reached an agreement that we would publish our research in the same journal at the same time. It was a shared recognition that this was significant, so it made sense to move the science forward together.”
The early work with KuDOS, and the development of olaparib as a lead compound, laid the foundation for the clinical trials that would follow – trials that would ultimately confirm the drug’s impact and change how BRCA-mutant cancers are treated.
Why olaparib succeeded, and others did not
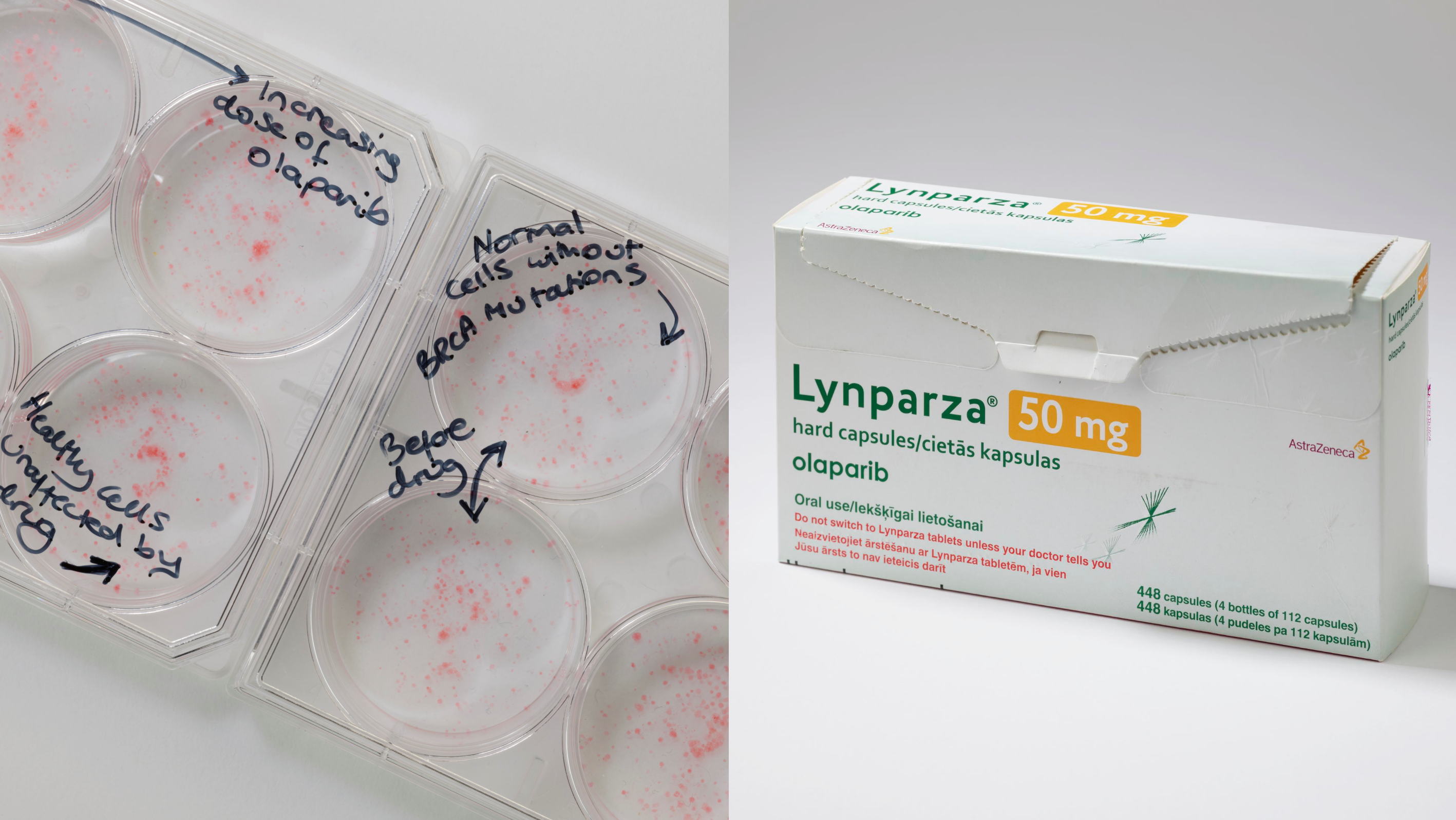
Image: Left: Plates showing the impact of olaparib on BRCA cancer cells and minimal impact of on healthy cells. Right: Lynparza, olaparib's brand name, drug packaging. Photo credit: Science Museum
Olaparib went on to demonstrate extraordinary promise in treating BRCA-mutant cancers and most recently has been given NICE recommendation for its use in advanced breast cancer. Many patients had already exhausted the existing treatment options, responded remarkably, with some experiencing sustained tumour shrinkage and extended periods of disease control.
Professor Lord said: “So, why did olaparib succeed when other targeted therapies struggled? Firstly, we understood the biology and knew who the drug would work for. That meant that the drug could be targeted to the right patients from the beginning.
“Secondly, patients tolerated olaparib far better than traditional chemotherapy – the side effect profile of olaparib was much milder, which meant people had a significantly better quality of life during treatment.”
The alignment between laboratory insight and patient selection proved crucial. Today, more than 140,000 patients have been treated with olaparib, and, in addition, three other PARP inhibitors are now routinely used to treat either breast, prostate, pancreatic or ovarian cancers. In no small part, these achievements have also been driven by key clinical trials testing PARP inhibitors carried out by the ICR’s Professor Johann De Bono and Professor Tutt. Approvals continue to expand into other cancer types as researchers uncover new settings where this approach is effective. These drugs have also proven effective for patients who have not inherited a BRCA mutation but have cancer with a BRCA-like profile – a concept known as ‘BRCAness’.
Resistance, combinations and a growing field
Despite the many successes to date, challenges remain. One of the biggest is drug resistance – when cancer cells evolve to survive even with a BRCA mutation and PARP being blocked.
Professor Lord said: “We’re currently working on ways to delay resistance or overcome it. We’ve also developed biomarkers that can tell us when resistance is starting to emerge, which could help inform clinicians to adjust treatment sooner.
“We’re also exploring new synthetic lethal combinations beyond BRCA. We’re not just applying this to BRCA-mutant cancers anymore. We’re investigating other DNA repair defects and identifying new targets. Using the concept of synthetic lethality to treat cancer how now been tried and tested with PARP inhibitors, and it has the potential to be widely applicable across many cancer types.
“Last year, I believe more than 400 papers were published on synthetic lethality in cancer, so it’s becoming a global field. I don’t know most of these authors – but I know where a lot of their work started!”
A career built on purpose

Image: Professor Chris Lord
Having spent the majority of his scientific career at the ICR – a place he says is uniquely equipped to translate discoveries from the lab into real-world treatments through the ICR’s unique partnership with The Royal Marsden NHS Foundation Trust – Professor Lord believes it’s one of the few institutions where such translational research is truly possible. Professor Lord said: “We’re set up to do translational research that actually makes an impact. Lots of places say they do that, but it’s hard. The ICR is good at it, and it’s a truly unique environment…and that’s why I’ve stayed.
“The breakthrough with PARP inhibitors was just as much personal as it was scientific. As a scientist, all you want is to make a discovery that’s genuinely useful. To have worked on something that has helped so many people – it’s an incredible feeling.”
Since 2005, Professor Lord’s research has remained focused on building that same kind of impact. He is searching for the next discovery that could change the trajectory of cancer treatment all over again.
Global effort, shared legacy
Although Professor Lord played a central role in the development of PARP inhibitors, he is quick to shift the spotlight onto the wider community that made it possible – from research collaborators and clinicians to patients and biotech partners.
Professor Lord said: “It’s easy to say, ‘this one person discovered it’, but that’s never the case. It takes an army, a village. I’ve got an empty box of olaparib on my desk, which I see as the tangible result of global collaboration – the end of one journey and the beginning of another.”
What started as a bold idea in DNA repair has since transformed the outlook for people with BRCA-related cancer and is paving the way for new therapies built on the same principle – using what is broken in cancer to destroy it.
.tmb-propic-md.jpg?Culture=en&sfvrsn=c25d2b2f_9)
 .
.
