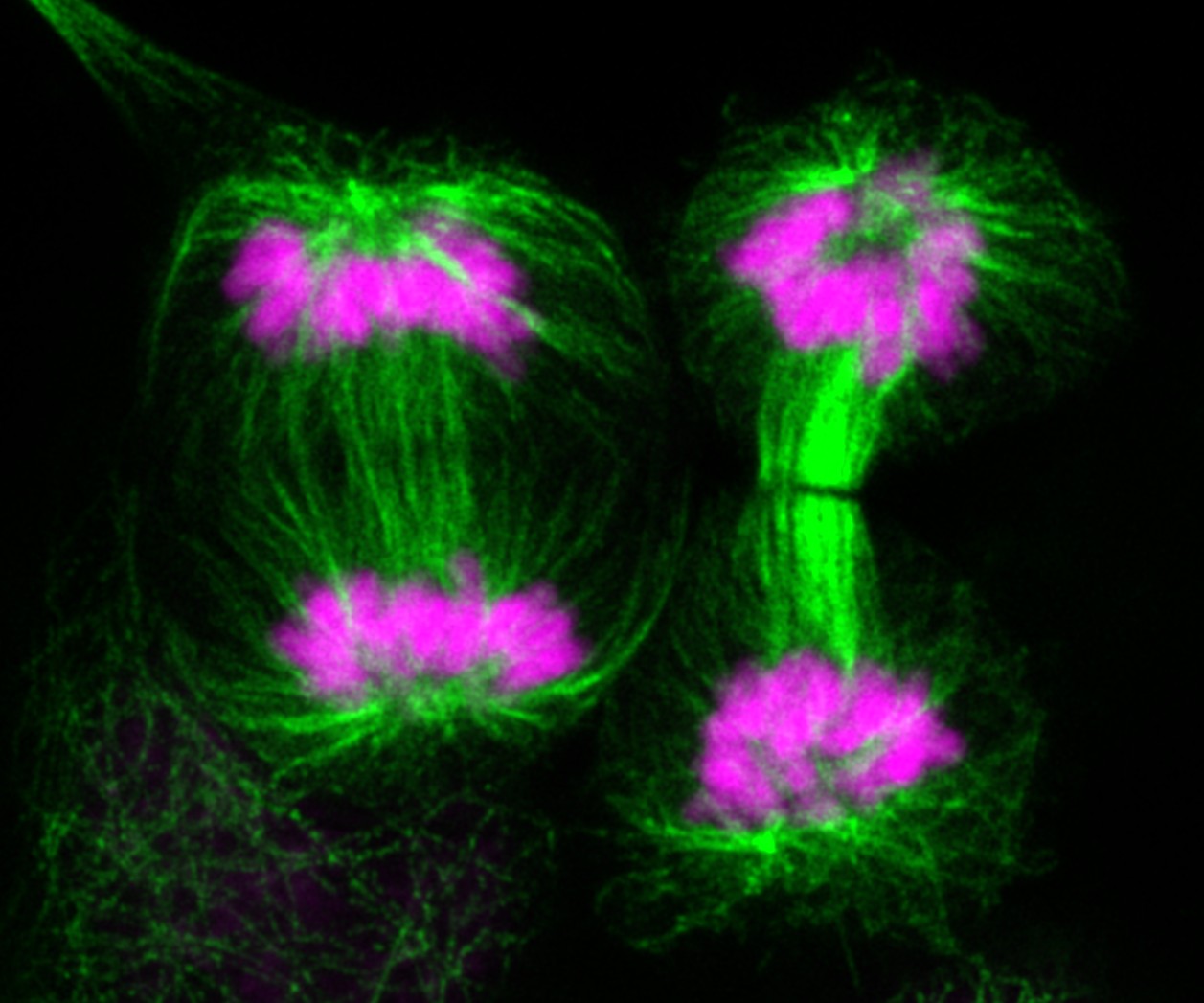
Centre for Children and Young People's Cancer
The Centre for Children and Young People's Cancer (CCC) serves as the focal point of basic, translational and clinical paediatric oncology research at the ICR and The Royal Marsden.
Our work involves close partnerships with academic and clinical institutions across the UK and around the world, particularly The Royal Marsden.
Our research is underpinned by Programmatic Funding from major research funders such as Cancer Research UK (including Cancer Grand Challenge awards), Stand Up 2 Cancer, ECMC, as well as more focused substantive awards from e.g. Brain Tumour Research, the Brain Tumour Charity, Sarcoma UK, Children’s Cancer and Leukaemia Group (CCLG) and Alice’s Arc Children’s Cancer Charity.
In addition, The Royal Marsden Cancer Charity provides significant funding to The Royal Marsden for the Oak Children and Young People's Drug Development Unit (OCYP-DDU) allowing us to turn the latest scientific advances into clinical benefits for young cancer patients.
The CCC has been launched to aggregate and expand existing activities in childhood and young peoples cancer at the ICR. This encompassed four previously established centres into a single over-arching structure, each retaining their existing activities and leadership.
Centre for Paediatric Oncology Experimental Medicine (POEM): leads Lou Chesler, Lynley Marshall. Encompasses the OCYP-DDU. Develops biomarker-driven clinical trials through expanding research infrastructure and academic / clinical training, and leadership in the national Stratified Medicine Paediatrics (SMPaeds) molecular diagnostics initiative and the Paediatric ECMC network, which has been tasked with the operational delivery of early phase clinical trials, translational research and innovation to children and young people around the UK.
Joint Sarcoma Research Centre (JSRC): leads Janet Shipley, Robin Jones. Brings together clinical, translational and preclinical sarcoma research across the ICR and The Royal Marsden, supported by the International Sarcoma Accelerator Consortium (Paul Huang).
CRUK Children’s Brain Tumour Centre of Excellence (CBTCE): leads Paul Workman, Richard Gilbertson (Cambridge). Links discovery science and innovative mouse modelling in paediatric brain tumours to focused medicinal chemistry expertise within the Centre for Cancer Drug Discovery (CCDD).
BTR PDHGG Centre of Excellence (BTRCoE): lead Chris Jones. Expands target validation and preclinical testing in PDHGG, with a direct link to review and clinical trial development within CONNECT; also supports joint PhD studentships across the ICR (Gabriela Kramer-Marek, Gary Newton) and The Royal Marsden (Julia Cockle).
How we research at this centre
The CCC serves an over-arching structure to co-ordinate our various activities across multiple childhood cancer types. It provides co-ordination of meetings and seminars of relevance to paediatric research, and provides a platform for resourcing common enabling technologies across our laboratories.
We aim to provide a national hub for research into children and young people's cancers, housing future grant-supported fellowships, studentships, lab rotations etc between the ICR and The Royal Marsden, and other institutions across the UK.
Through our joint institutions, this will also facilitate an increase the number of hypothesis-driven, first-in-child/young person and other early phase clinical studies of molecularly targeted anticancer drugs through our own Centre for Cancer Drug Discovery’s programmes, and by increasing the availability of new drugs for investigation by maintaining strong and unified links with pharmaceutical and biotech companies, and our leadership activity within national and international consortia.
Well-annotated data and AI-driven discovery
Childhood cancers are rare, resulting in few samples for analysis, small cohorts of patients in clinical trials, and fragmented datasets in the public domain. They also comprise a remarkable diversity of sub-types, providing challenges for translational biology, but also opportunities for improved molecular diagnostics.
We will build upon national initiatives (SMPaeds1/2), and individual retrospective and clinical trials cohorts to link with the Integrated Pathology Unit (IPU) and Data Science Team (DST) to generate and exploit multi-omic and single cell atlases of childhood cancer and their immune microenvironment (with the Centre for Translational Immunology, CTI), with high-quality clinical annotation, to better classify these tumours, map their developmental origins, and track their evolution under therapy.
Novel therapeutic approaches
Childhood cancers have their origins in development, whereby stalled maturation programmes result in cells locked in a progenitor-like state. They have bespoke drug targets which include developmentally-restricted transcription factors, and epigenetic modifiers which control cell plasticity, however these attract little pharma interest due to small patient populations, but also the limited availability of models and difficulty in running clinical trials.
We will link with the Centres for Target Validation (CTV) and Protein Degradation (CPD) to initiate specific drug discovery projects in this area (through the CBTCE where appropriate), and exploit the new Centre for In Vivo Modelling (CIVM) to assist in development of more physiologically relevant models of disease subtypes (including relapse/metastasis) to resource this.
Forward / reverse clinical translation
Via the NHSE WGS programme for all childhood cancer patients, and via SMPaeds2, we will increase the number of patients screened for biological molecular markers to guide entry into relevant clinical trials.
We will continue to develop more sophisticated and more efficient clinical trial designs such as platform/basket trials that require fewer patients to answer relevant trial questions, such as CONNECT-TarGeT and ITCC-ELICIT in PDHGG.
We will aim to learn from every patient on trial through implementation of co-clinical trials platforms using in vitro and in vivo patient avatars to inform biologically-rational treatment choices and resistance modelling.
We will incorporate minimally invasive translational laboratory and functional imaging biomarkers into early clinical trials wherever possible, to help understand disease response (or non-response) to new therapies, thus helping our iterative lifecycle style approach to paediatric cancer drug development.
Researchers at the ICR and The Royal Marsden hold key leadership roles in national and international clinical trials consortia (covering early and late phase studies), with direct evidence of translating laboratory discoveries to the clinic (e.g. Children’s Cancer and Leukaemia Group (CCLG), NIHR Clinical Study Groups, The Experimental Cancer Medicines Centre (ECMC) Network, International Society for Paediatric Oncology (SIOP) Europe including the SIOPE-Brain Tumour Group and SIOPEN (Neuroblastoma) Group, New Approaches to Neuroblastoma Therapy (NANT), European paediatric Soft tissue sarcoma Study Group (EpSSG), Collaborative Network for Neuro-oncology Clinical Trials (CONNECT), the UK Paediatric Transplant and Cell Therapy Network (‘ATICUS’) and the Innovative Therapies for Children with Cancer (ITCC) Consortium.
The ITCC comprises 62 clinical research centres and 25 academic research laboratories from across Europe (17 countries) with a focus on translating innovative laboratory discoveries to the clinic and developing novel therapies for the treatment of paediatric and adolescent cancers.
As part of an initially EU IMI-2 funded project, the ICR is a co-founder of a non-profit gGmbH spin-out company ITCC-P4 (ITCC Paediatric Preclinical Proof-of-Concept Platform), which offers preclinical testing on >400 childhood cancer models, many of which were developed by the ICR's researchers using samples from patients at The Royal Marsden. Faculty at the ICR are contracted entity experts of the gGmbH, providing an advisory role for experiment interpretation and ongoing strategy.
The ITCC-Brain Committee has also recently been awarded a €2M grant from Fight Kids Cancer to establish the Translational Accelerator Platform taking advantage of the ITCC-P4 offering, with the ICR forming the European PDHGG expert hub.
ICR Members

Sir Mel Greaves
Group Leader
Biology of Childhood Leukaemia

Professor Chris Jones
Head of Division
Glioma.tmb-propic-md.jpg?Culture=en&sfvrsn=c25d2b2f_9)
Professor Louis Chesler
Clinical Senior Lecturer/Group Leader
Paediatric Solid Tumour Biology and Therapeutics
Professor Paul Huang
Group Leader
Molecular and Systems Oncology
Dr Sally George
Group Leader
Developmental Oncology
Dr Gary Newton
Group Leader
Medicinal Chemistry 3Honorary Faculty Members

Dr Henry Mandeville
Honorary Faculty

Dr Lynley Marshall
Honorary Appointment
Sarcoma Clinical Trials in Children and Young People
Dr Julia Chisholm
Honorary Faculty
Sarcoma Clinical Trials in Children and Young People
Dr Fernando Carceller
Associate Honorary Faculty
Paediatric & Adolescent Neuro-Oncology and Drug Development (Carceller)
Dr Caroline Furness
Honorary Faculty
Paediatric Stem Cell Transplant
Dr Julia Cockle
Honorary Appointment
GliomaAdditional researchers
- Mary Taj
- Elsje Van Rijswijk
- Sanjay Tewari
- Robin Dowse
- Elizabeth Corley
- Menna Shamma
- Anna-Karenia Anderson
- Assunta Albanese
Our publications
- Liu I, Cruzeiro GAV, Bjerke L, Rogers R, +61 authors, Monje M, Jones C* and Filbin M* (2024) “GABAergic neuronal lineage development determines clinically actionable targets in diffuse hemispheric glioma, H3G34-mutant” Cancer Cell 42(9):1528-1548 [PMID: 39232581]
- Izquierdo E, Carvalho DM, Mackay A, +30 authors, Hargrave D and Jones C (2021) “DIPG harbour alterations targetable by MEK inhibitors, with acquired resistance mechanisms overcome by combinatorial inhibition" Cancer Discov 12(3):712-729 [PMID: 34737188]
- Carvalho DM, +28 authors, Carceller F and Jones C (2021) “Repurposing vandetanib plus everolimus for the treatment of ACVR1 mutant DIPG” Cancer Discov 12(2):416-431 [PMID: 34551970]
- Clarke M, Mackay A, Ismer B, +100 authors, Ellison DW*, Jacques TS*, Jones DTW* and Jones C* (2020) “Infant high grade gliomas comprise multiple subgroups characterised by novel targetable gene fusions and favourable coutcomes” Cancer Discov 10(7):942-963 [PMID: 32238360]
- Vinci M, +33 authors, and Jones C (2018) “Functional diversity and co-operativity between subclonal populations of paediatric glioblastoma and diffuse intrinsic pontine glioma cells” Nature Med 24(8):1204-1215 [PMID: 29967352]
- Mackay A, +24 authors, Jaspan T, Varlet P and Jones C (2018) “Molecular, pathological, radiological and immune profiling of non-brainstem paediatric high grade glioma from the HERBY phase II randomised trial” Cancer Cell 33(5):829-842 [PMID: 29763623]
- Mackay A, Burford A, Carvalho D, Izquierdo E, +50 authors, Jones C (2017) “Integrated molecular meta-analysis of 1000 pediatric high grade glioma and diffuse intrinsic pontine glioma” Cancer Cell 32(4):520-537 [PMID: 28966033]
- Taylor KR*, Mackay A*, +19 authors, Jones C* and Grill J* (2014) “Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma” Nature Genet 46(5):457-461 [PMID: 24705252]
- Bjerke L, Mackay A, +16 authors and Jones C (2013) “Histone H3.3 mutations drive paediatric glioblastoma through upregulation of MYCN” Cancer Discov 3(5):512-519 [PMID: 23539269]
- Paugh BS*, Qu C*, Jones C*, +13 authors and Baker SJ (2010) “Integrated molecular genetic profiling of paediatric high grade gliomas reveals key differences with the adult disease” J Clin Oncol 28(18):3061-3068 [PMID: 20479398]
News and discoveries
.jpg?sfvrsn=21a64ae3_1)
New cancer test could predict, up to 10 years in advance, when treatment will be needed

The ICR and The Royal Marsden team up with PureGym to support men with advanced prostate cancer to be more active

Subtle chemical tweak helps determine whether cells grow, potentially affecting cancer risk