The team studies a broad range of oncolytic virotherapy agents, including reovirus type 3 (Dearing), herpes simplex virus type 1 (T-VEC, Replimune agents RP1, RP2), vaccinia virus, coxsackie A21 virus, Maraba virus and Newcastle disease virus). These agents are able to grow in – and kill – cancerous, but not normal, cells.
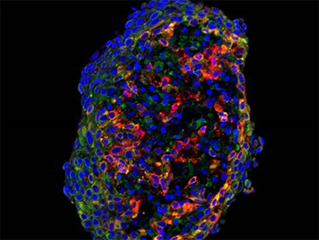
Image right: Tumour spheroids
Some of these so-called oncolytic viruses have naturally evolved to grow preferentially in cancer cells because of the cells’ specific genetic defects, while others have been genetically engineered to grow selectively in cancer cells.
This aspect of the team’s research is led by Dr Joan Kyula-Currie and Victoria Roulstone whose recent work has focused on harnessing the potential of oncolyitic viruses to trigger anti-tumour immune responses, alone or in combination with other therapeutic modalities.
Professor Harrington hopes new treatments using these viruses will improve patients’ cure rates and have fewer side-effects than current therapies. Professor Harrington’s team has completed a large number of translational clinical trials of oncolytic virotherapies and he was UK Principal Investigator on the pivotal phase III OPTiM trial that led to FDA approval of T-VEC.
The team’s work on targeted radiosensitisers involves detailed mechanistic studies of the effects of small molecules that can modulate cellular responses to DNA damage. In particular, drugs that target the G2/M phase of the cell cycle are seen as having great potential for enhancing anti-tumour immune responses.
This component of the team’s research is led by Dr Martin McLaughlin and Dr Emmanuel Patin. The team has expertise in researching inhibitors of HSP90, Chk1, ATR and DNA-PK and most of the recent work has focussed on characterising phenotypic and functional changes in immune cell infiltrates in response to combinations of targeted radiosensitisers and ionising radiation.
These studies have been translated into clinical trials, including the phase I PATRIOT study of the ATR inhibitor, AZD6738. Further research will expand our understanding of the effects of combining radiation, DNA damage response modifiers and immune checkpoint inhibition.
In recent years, the team has developed significant expertise in the field of thyroid cancer, under the leadership of Dr Malin Pedersen. This work covers a range of research projects evaluating the therapeutic potential of small molecule inhibitors (B-Rafi, Pan-Rafi), oncolytic virotherapies and immune checkpoint inhibition. These agents are under test as stand-alone therapies, but more commonly in the context of combinatorial regimens.
Professor Harrington’s team is also active in research projects that exploit surgical models to understand the potential benefits of virus therapies. The team’s work on isolated limp perfusion (ILP), in collaboration with Mr Andrew Hayes (Consultant Surgeon, RMH), has used a rat sarcoma model to demonstrate that intravascular delivery of vaccinia virus can augment the therapeutic gains from melphalan and TNF-alpha.
Furthermore, the team’s work has shown that a combination regimen of viral ILP, surgery, radiotherapy and immune checkpoint blockade can protect against systemic relapse of disease. These findings have been translated into a phase I clinical trial (TITAN) of ILP plus T-VEC (under the leadership of Andrew Hayes).
In addition, by using a novel rat model of free flap transfer, the team has evaluated the role of lentiviral gene delivery as a means of mitigating the damaging effects of radiotherapy in normal tissues (under the leadership of Mr Aadil Khan).
Gene therapy-mediated silencing of connective tissue growth factor (CTGF) and overexpression of mitochondrial superoxide dismutase (Mn-SOD) has been shown to reduce radiation-induced damage in a rat superficial inferior epigastric flap model. Further studies are evaluating the roles of immune cells in mediating radiation-induced fibrosis.
Much of Professor Harrington’s laboratory work is immediately translated into clinical trials at the Royal Marsden NHS Foundation Trust, most often in patients with head and neck cancers, and melanomas.
Professor Harrington says that the ICR’s unique partnership with the Royal Marsden allows him to conduct innovative laboratory research and apply it in the clinical setting, achieving “real patient benefit” in the shortest possible time frames.