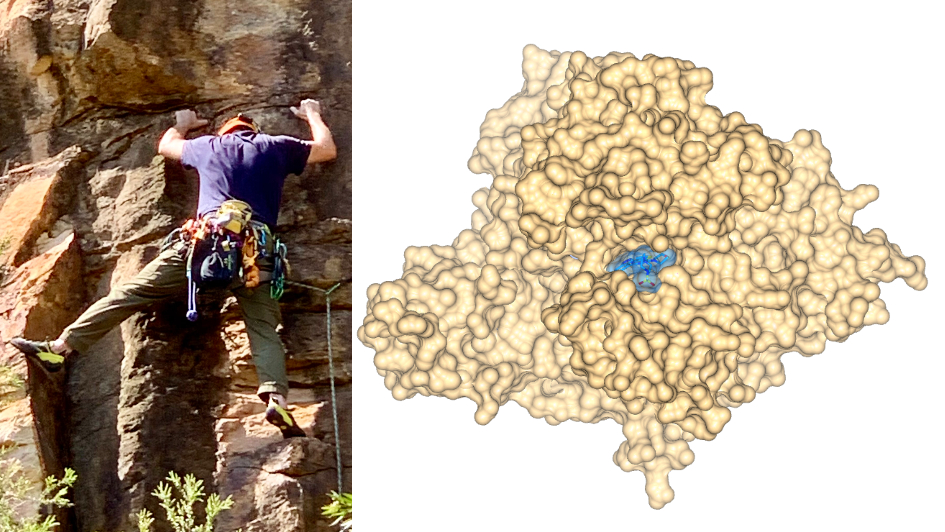
Image: Just as a climber inspects the ledges and crevices in a rock face, drugging a disease target requires an assessment of its molecular structure and druggability. Credit: Kgbo (left) and Dr Rob van Montfort, ICR (right)
As a drug discovery scientist I think a lot about drug targets – which are the molecules in the cell, usually proteins, that drugs act on to exert their therapeutic effects. Choosing the molecular targets to go after is probably the most important thing that drug hunters do – since if we get this wrong we will consume expensive resources for a very long time, only to fail at the end.
Experience has shown that drug targets are not all equal, although this is not widely understood. To explain why drug targets vary in how technically doable or difficult they are, I think it’s useful to use a metaphor.
Imagine you are a mountain climber. You assess your next ascent. You are initially daunted by the near vertical surface. But on closer inspection you find small ledges, crevices and indentations in the rock face that will allow your hands and feet to gain purchase. You can plan your climb based on these bumps, nooks and crannies.
But now imagine instead that you find a vertical wall of rock that is smooth with no opportunities to gain a grip or foothold. There would be no way that you would contemplate such a climb, however experienced you were.
Of course, ascents are not black and white – either easily climbable or impossible to attempt. They vary in difficulty, ranging from the easy – perhaps with less steep a slope and many helpful and well-placed protuberances and indentations making them suitable even to novice climbers – through to more challenging ascents, and then all the way to the almost certainly impossible conquest.
Climbing is a good analogy for the discovery of targeted, ‘small molecule’ drugs. In order to exert an effect on a target protein that has been demonstrated to be important for the disease of interest, a drug needs first to bind to the protein surface.
And just as with the different climbability levels of rock faces, protein targets vary enormously in their ‘druggability’. Proteins that are relatively easy to drug are those with clearly defined pockets or cavities into which a small molecule can attach, such as the active site of an enzyme that is used to bind its biological substrate. Other druggable proteins feature a groove or channel that is involved in forming an interaction with another protein and into which a drug can squeeze.
In the case of drugging a protein, binding of a small molecule to a single druggable cavity or channel can be sufficient for success. Knowledge of the 3D structure and druggable cavities of a protein powerfully enables structure-based drug design. Initial binders are usually found by screening collections of drug-like small molecules or tinier fragments.
Druggable proteome
Based on knowledge or prediction of their 3D structures, it’s possible to estimate how many human proteins are likely to be druggable. In fact my colleagues and I have done this using the state-of-the-art methods and data contained in our ICR canSAR BLACK knowledgebase – developed by Professor Bissan Al-Lazikani and colleagues and described in our most recently published update.
This identifies potential binding cavities and assesses the druggability of them, using machine learning and AI, according to a number of molecular features – analogous to assessing the features of individual footholds and handholds on our rock face that cause them to offer differing degrees of grip.
The total number of human proteins – known as the reference proteome – is 20,375. Using our range of powerful predictive methods, based on 3D protein structure and past precedent, we calculate that 5,160 proteins (25% of the total) contain cavities that are estimated to be druggable with ‘drug-like’ small molecules. This number doubles to 10,248 (50%) if we include potential for antibody-based drugs acting on protein targets located on the outside of cells.
However, only a much smaller fraction of human proteins – estimated at around 670 or 3% of the proteome – are currently drugged by medicines that are actually approved and available to treat patients.
Climbers looking at such numbers would see enormous potential to conquer new heights.
Why is the fraction of the human proteome that has been successfully drugged so low?
The first reason is that not all human proteins are causally involved in disease. Only where there is strong, robust evidence – referred to as target validation – that modifying the function of the protein will alter the course of a disease, or ameliorate symptoms, will it be suitable for a drug discovery project.
It has been estimated that of the 20,000 or so protein-coding genes, around 10% are likely to be disease-modifying. It is however possible that there are many proteins for which a role in the initiation and progression of disease has yet to be discovered and validated.
But then there is the technical druggability challenge – not all proteins involved in disease will be suitable for binding a drug-like small molecule (or antibody), at least using current technology.
Good targets for drug discovery are those that are both involved in disease and also readily or potentially druggable.
Looking at the approved drugs that act on disease-modifying human proteins, certain target families stand out. These include protein kinases, PI3 kinases, phophodiesterases, metallopeptidases, proteinases, G-protein coupled receptors, ion channels and nuclear hormone receptors.
Part of the reason that particular classes of drug are represented so heavily is because they have been found to be readily druggable and so had the most attention paid to them by drug discovery researchers. Other disease targets have proved much more difficult to drug, or there has been less effort to drug them.
Some time ago, Bissan, our colleagues and I published an objective, large-scale assessment of the protein products of 479 genes associated with cancer, including an analysis of the druggability of their protein products. A number of the cancer protein targets we highlighted as druggable have since been progressed into drug discovery and development projects.
Many of the targets of modern precision cancer medicine are kinases, where the drug binds into a pocket on the protein normally occupied by the natural small molecule ATP, which is needed for kinase activity. Examples are gefitinib, imatinib and capivasertib.
In other cases, the target is a nuclear transcription factor involved in controlling the activity of genes, and the drug binds into a cavity used by a natural ligand that normally regulates the target – as with the oestrogens and androgens acting on their respective transcription factor receptors.
Some particularly common and important cancer-causing proteins have traditionally been highlighted as intractable to small-molecule inhibitors, including drivers such as MYC, mutant RAS, mutant p53, fusion transcription factors and androgen receptor (AR) variant 7.
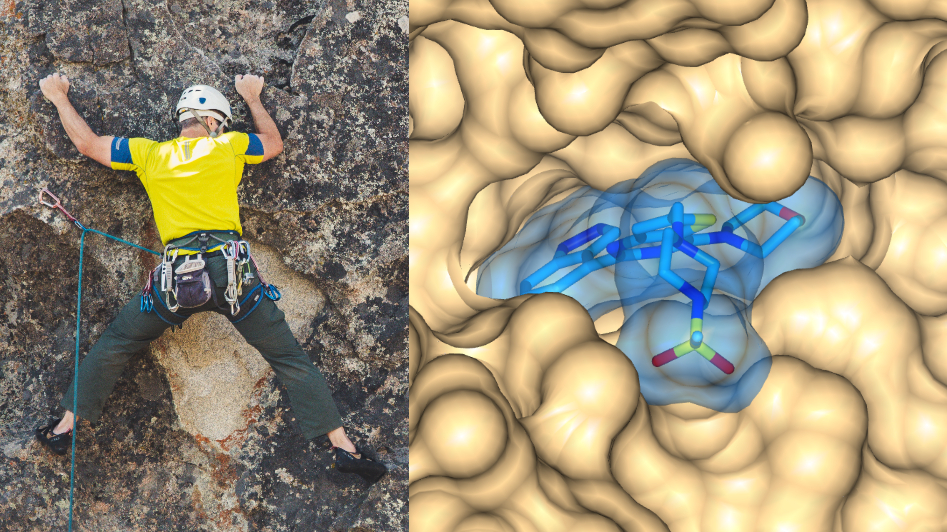
Image: Some proteins have clearly defined pockets or cavities into which a small molecule can attach – just as a climber grips an indentation in a rock face. Credit: Brett Sayles (left) and Dr Rob van Montfort, ICR (right)
Blocking protein-protein interactions
Targets whose disease-causing activity is mediated by binding to other proteins, and where a drug would need to block the protein-protein interaction, have historically been viewed as especially difficult to drug.
But more recently, researchers have found ingenious ways of drugging the interactions between various proteins that play a key role in cancer – for example between p53 and MDM2, and between the BCL2 family of survival proteins and their binding partners such as BAX and BAD. Drugs are now on the market for cancer treatment that act by disrupting these protein-protein interactions.
The successful drugging of BCL2 family protein-protein interactions was very challenging but was achieved with high-quality science, state-of-the art technology, and step-by-step improvement by a big research team active over a sustained period.
The protein product of the mutant KRAS cancer gene is another example of a target that for a long time was considered ‘undruggable’ because the affinity of the natural ligand in the active site pocket is so tight that it prevents drug binding. However, very recently small-molecule drugs targeting mutant KRAS, such as sotorasib, have progressed to the clinic and shown therapeutic activity.
In the case of sotorasib and similarly acting candidate drugs now undergoing clinical trial, the drugging of a particular cancer-causing mutant form of KRAS, known as G12C, was based on the discovery of small molecules which bind in a previously unappreciated pocket and interact irreversibly with the closely located cysteine amino acid – which is present in the G12C mutant KRAS but not the non-mutant protein – thus providing specificity towards cancer versus normal cells.
Additional recent approaches to drugging RAS involve perturbing the productive protein-protein interactions with partner proteins. ICR researchers Dr Olivia Rossanese and Professor Terry Rabbits have each been instrumental independently in these discoveries.
Perseverance and ingenuity
These examples show that even some extremely challenging drug targets can be tackled successfully where researchers show sufficient perseverance and ingenuity, especially exploiting knowledge of 3D protein structure.
So it’s important to stress to non-specialists that drug discovery is not just about finding a gene or protein with a clear involvement in a disease. It’s also essential to consider whether – and if so to what extent – the disease target is druggable. Some targets are very challenging. At the same time, we should not underestimate the ability of human ingenuity and innovative technology to overcome a tough druggability challenge.
It’s all about being aware of the size of that challenge for each individual target and tailoring the drug discovery approach accordingly. If a protein is readily druggable then conventional drug discovery approaches should work well. But if the druggability analysis shows that the target is a major technical challenge then researchers can decide either to walk away or to apply extra ingenuity and resources.
I started this post by likening the concept of druggability to climbing rock faces with different levels of difficulty. I do think it’s important to keep pushing the boundaries of druggability, and certainly not to give up on the tougher targets or discourage ingenuity or novel technical breakthroughs.
Sharing unsuccessful stories
There’s also another key point I’d like to stress. Drug discovery builds on past successes and learns from earlier failures. So it’s essential that researchers publish their experiences in tackling the hard-to-drug targets – including the unsuccessful as well as the successful stories.
The sharing of cumulative experience can help in managing the expectations of biological researchers who are enthusiastic to run small-molecule screens against their favourite protein of interest. They must appreciate that this might turn out to be a relatively easy climb, a more difficult one, or even impossible.
And as with climbing, a good hard look at the target is essential preparation!
Note: The images in the blog post show the X-ray co-crystal structure of the clinical class I PI3 kinase inhibitor pictilisib/GDC-941 bound to the ATP pocket of human PI3 kinase gamma (PDB 3DBS).
comments powered by