Over a hundred years ago William Coley made the striking observation that tumours underwent spontaneous and sustained regression in patients with severe postoperative infection. This led to the finding that the body’s immune system makes attempts to eliminate cancer cells from the body – as it would any foreign invader.
As part of its attack, the immune system promotes inflammation – which is the cause of fever, pain and swelling in people who are sick or injured. But while in many people this attack by immune cells on cancer may be initially successful, sometimes cancer cells survive this onslaught, and even evolve the ability to thrive in the presence of inflammation. In fact, cancers have been described as ‘wounds that never heal’.
That inflammation can be a cause of cancer has largely stopped the use of pro-inflammatory agents, such as TNFalpha, as anti-cancer therapeutics. But interest into using the body’s own inflammatory response to fight cancer has been reinvigorated by the promising results of immunotherapies. Knowing why some cancers can be stopped by inflammation and pro-inflammatory cytokines, and why others feed on it, is critical in order to decide how to treat cancers effectively.
In new work published in Molecular Systems Biology, we have shown that the shape of breast cancer cells and their ‘microenvironment’ affects how they will respond to inflammation. By using robotic microscopy and automated algorithms to measure the shape of hundreds of thousands of different breast cancer cells – these algorithms are similar to those used in facial recognition programs used by Facebook – we asked whether there was a connection between cell shape and the activity of NF-kappaB, a transcription factor that is turned on by cells when they are exposed to TNFalpha. NF-kappaB is also a well-established oncogene and cancer driver.
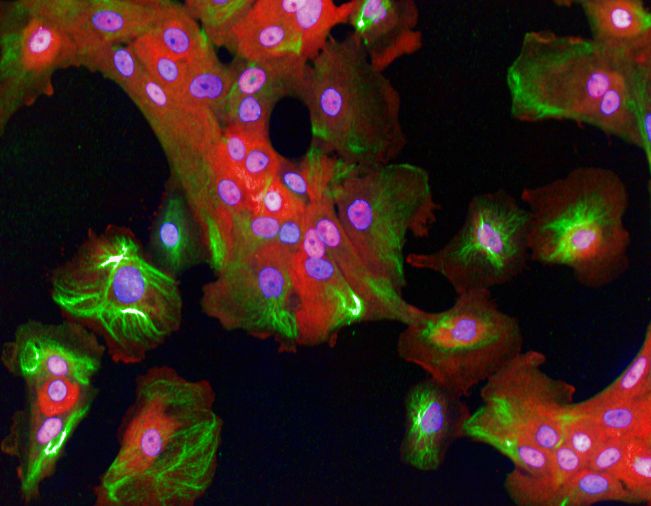 Breast cells responding to inflammatory signals
Breast cells responding to inflammatory signals
Cell shape
We found that the shape of the cell affects NF-kappaB activation. Specifically we found that TNFapha is less effective at turning on NF-kappaB in cells that have a shape similar to normal undamaged epithelial cells. However, TNFalpha strongly activates NF-kB in cells with a different shape, such as those at the edges of tissues that have been wounded. We also found that the local environment of a cell decides how strongly NF-kB responds to inflammation. NF-kB is weakly activated in cells with lots of neighbours, but strongly activated in cells with few neighbours. This work implies that the shape of a tumour cell is an important factor is determining whether inflammation will promote or inhibit cancer progression.
Interestingly NF-kappa activity in response to TNFalpha is typically lower in ‘luminal’ types of breast cancer cells, because they have a shape that is similar to normal epithelial cells. In contrast, in many ‘basal' breast cancer cells TNFalpha induces a stronger activation of NF-kappaB than their luminal counterparts because their shape is quite different.
Luminal breast cancers are much more effectively treated than basal breast cancers, and our work suggests that differences in cell shape, and thus how cells respond to inflammation, may underlie some of the differences between basal and luminal cells.
Importantly, we found that by using mechanical, chemical, or genetic means to change cell shape, we could affect NF-kappaB activation in cancer cells. So manipulation of cell shape may be completely novel way to make tumour cells more sensitive to treatment. For example, changing cell shape may be a way to do a ‘makeover’ on tumours that thrive on inflammation, and turn them into those that can be more easily eliminated by the immune systems or pro-inflammatory compounds.
comments powered by